Addressing Non-Adherence In Patients With Diabetes
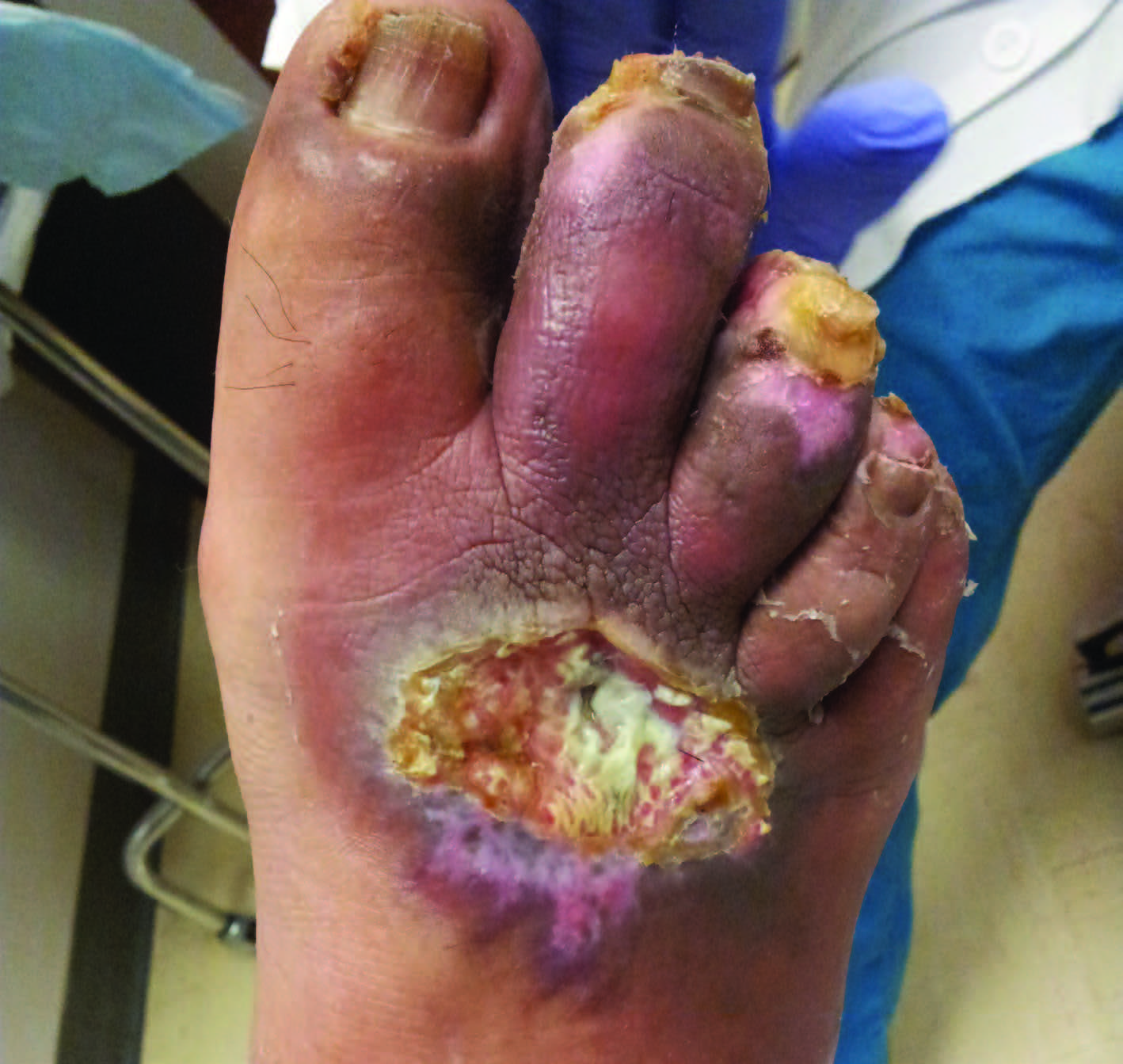
There are reportedly 463 million people worldwide living with diabetes and that number is expected to increase to 700 million by 2045.1 Based on the most recent estimates from the Centers for Disease Control and Prevention (CDC), 30.3 million people in the United States have diabetes, including 7.2 million who are undiagnosed.2 Up to 25 percent of patients with diabetes will develop a foot ulcer in their lifetime and one in five of these patients will require an amputation.3
Treatment and limb preservation are ongoing struggles in patients with diabetic foot ulcers. What looks promising at one visit can quickly deteriorate into a life-threatening situation. Whether it is a particular offloading device, wound dressing, diabetes medication or antibiotic, there are countless ways in which treatment plans and protocols can deviate from the intended outcomes. As countless wound care physicians and surgeons have experienced throughout the years, the best laid plans often go awry.
Accordingly, let us take a closer look at patient adherence or patient concordance, and steps we can take to strengthen the role and autonomy of patients. For too long, patients endured much of the blame for less desirable outcomes. Now providers are increasingly shouldering accountability for poor communication and a lack of empathy. Moving forward, providers should view patients as partners rather than obstacles in limb preservation.
Rethinking The Term ‘Compliance’ And Understanding The Factors That Contribute To ‘Adherence’
There is tremendous interest in the relationship between chronic disease management and compliance. Only recently has the scientific community more widely accepted the term adherence. Traditionally, “compliance” and its negative counterpart “non-compliance” have described the level or extent to which a patient followed a recommended treatment plan. Further divided into primary and secondary categories, primary non-compliance refers to a scenario in which a patient fails to have medication dispensed whereas secondary non-compliance occurs when the pharmacy dispenses the medication, but the patient does not take it as directed. Intentional non-compliance occurs when a patient rejects a doctor’s diagnosis or treatment plan. This may be related to social, demographic, psychological and clinical factors.
While the medical profession used these terms relatively freely and interchangeably in the past, they often carry a negative connotation. Furthermore, compliance may correlate with the belief that patients must comply, without any recourse or alternative, and that non-compliance is exclusively the fault of the patient. In reality, there are many potential reasons why a patient may not follow a recommended treatment plan, such as a previous adverse reaction to a prescribed medication or other cultural beliefs.4
As defined by the World Health Organization (WHO), adherence refers to “the extent to which a person’s behaviour … corresponds with agreed recommendations from (the) health care provider.”5 This is in contrast to compliance, which is based on an assumption of “joint responsibility for health outcomes” between patients and their health care teams.6
In order to better understand factors contributing to patient non-adherence, the World Health Organization proposed five dimensions of adherence that should factor into the treatment strategy. These dimensions include: social and economic factors; therapy-related factors; patient-related factors; health system-related factors; and condition-related factors.5 It is not difficult to incorporate these factors into the treatment of patients with diabetes, particularly those with DFUs. While our specialized training makes it easy to regard a basic dressing as “simple,” it behooves providers to understand the complete spectrum of challenges faced by our patients and their caretakers.
In order to maximize ulcer-free days and decrease amputations, we need a comprehensive approach to the treatment of the diabetic foot. In addition, the most recent guidelines from the International Working Group on the Diabetic Foot stress patient education and communication as key tenets in the prevention of DFUs and their recurrence.7
Wound Care Dressings And Adherence: What You Should Know
When it comes to wound dressings, it is critical to account for patient adherence. Certainly, other key factors include the appearance of the wound and periwound area, location, size, depth, exudate type and amount, underlying vascular status, and risk for infection along with the ability of the dressing to remain in place for a given period of time and adequately absorb drainage. In addition, one must take into account cost-effectiveness, ease and comfort of application and removal, and compatibility with an associated offloading device.8
While it is important to educate patients about treatment plans, research has shown that education alone does not produce “clinically relevant reductions in ulcer or amputation incidence,” and providers should “focus on clear and simple messages that are tailored to an individual’s lifestyle, health literacy level and understanding of accessing care.”6 In addressing barriers to adherence, Devine and colleagues state, “development of novel treatments may be helpful, but we may get more bang for the buck by (ensuring) that the medications we already have are properly used.”9 In certain cases (i.e. when patients have high risk for infection, copious exudate and/or an inability to travel to and from appointments), we must rely on our patients and their caretakers to perform dressing changes. Written instructions, in-office demonstrations and even video may provide a great deal of assistance. Taking the time to understand our patients’ needs and ability could mean the difference between healing and amputation.
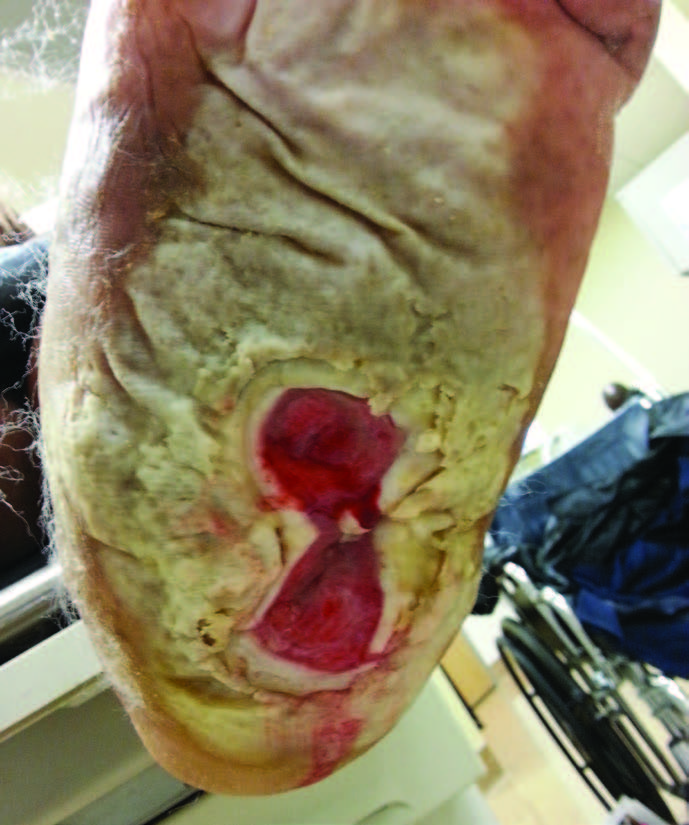
For complex wounds, many advanced wound care dressings, including negative pressure wound therapy (NPWT) as well as certain cellular and/or tissue-based products, demonstrate great promise in healing DFUs and preventing amputations in addition to contributing to adherence.10,11 Providers and their teams apply and change these dressings, thus obviating the need for patients to do so and the associated risks.
Assessing The Adherence Impact With Offloading Modalities
Patients with lower extremity complications of diabetes, including peripheral neuropathy, lack the “gift of pain” and may not sense when there is an emerging foot problem.12 In the same regard, patients with foot ulcers may not adhere to certain offloading recommendations due to a perceived lack of pain. Thus, providers must adopt strategies and form partnerships with patients in order to find the right offloading method.
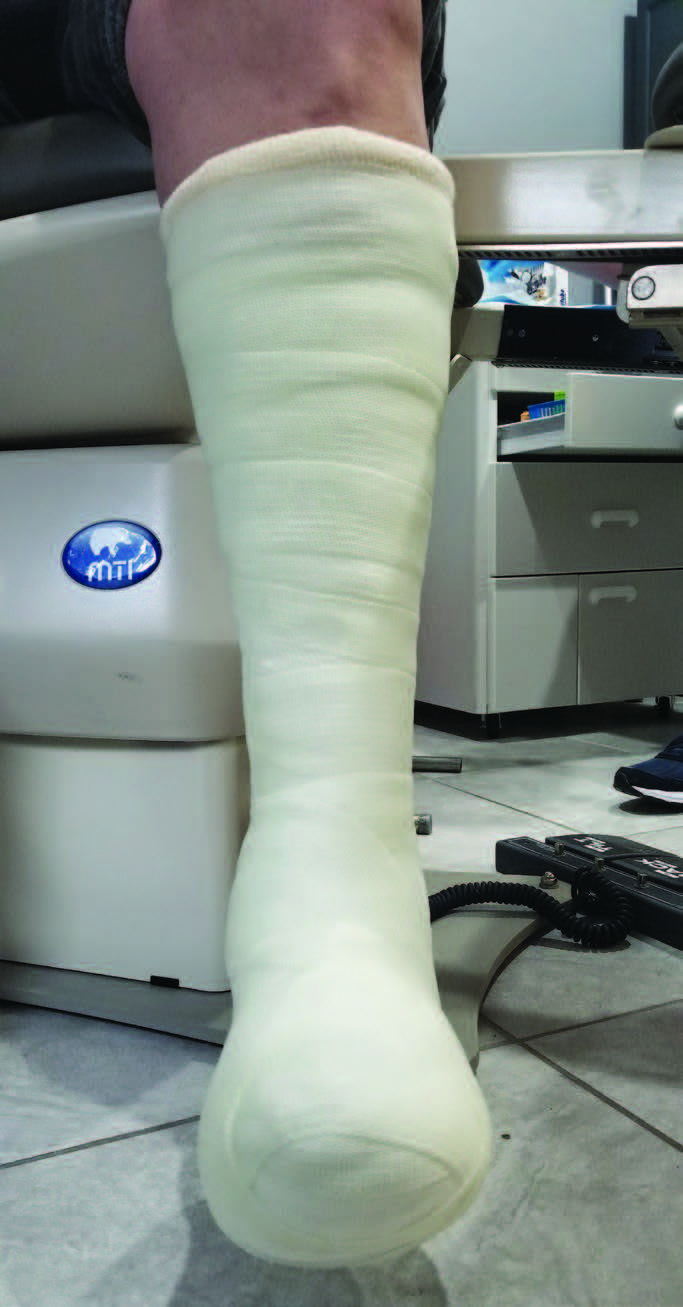
Total contact casting, a “gold standard” in offloading DFUs, can reduce pressure by up to 84 to 92 percent at the site of a plantar neuropathic ulcer and heals most DFUs within six to eight weeks.13 In a systematic review by Bus and colleagues, the authors found that non-removable offloading, such as TCC, is more effective than removable devices for healing neuropathic foot ulcers.14 However, in a large multicenter study, Wu and team found that only 1.7 percent of 895 centers actively treating DFUs used TCC for the majority of cases.15 Cited factors contributing to the lack of use of TCC included patient tolerance, time needed to apply and remove the cast, material costs, and reimbursement challenges.15
Yet one of the most important attributes of TCC is ensuring proper patient adherence due to the modality’s irremovability.16 In contrast, removable cast walkers allow for more frequent wound and skin inspection and dressing application, and, in some cases, provide nearly as much pressure reduction as the TCC.17 However, they are not as effective at healing DFUs due to issues with adherence.18 A proposed alternative, the instant total contact cast (iTCC), demonstrates healing results equivalent to TCC and may be more tolerable to patients. The iTCC consists of a removable cast walker wrapped with cohesive bandage or fiberglass, thus rendering the device irremovable.19
A more recent version of the iTCC (Optima Diab, Optima Molliter) has a rigid rocker sole and a modular insole with three layers of varying stiffness that providers can alter depending on ulcer location. There is also a rigid posterior brace and a plastic lace that render the boot irremovable. In a 2007 randomized, prospective study, Piagessi and colleagues noted the Optima Diab walker was as safe and effective as TCC in managing DFUs.20 A larger study by the same group in 2016 found similar results but also found no difference between the boot and TCC “irrespective of removability.”21 In both studies, researchers observed better applicability (taking the device on and off) and lower costs associated with the Optima Diab walker.20,21
Can Remote Temperature And Pressure Monitoring Technologies Reinvent Patient Adherence?
Recent advances in technology, including remote temperature and pressure monitoring and wearable textiles, may represent true “game-changers” in the management of patients with diabetes.
One example is the recent partnership between the aforementioned Optima Molliter and Sensoria Health, which aims to combine a state-of-the-art offloading device with real-time sensors and remote monitoring capabilities. The Motus Smart, powered by a Sensoria® offloading boot, uses a real-time mobile app and detects patient behavior. An alert system and dashboard can inform caregivers and clinicians of these behaviors, allowing for immediate intervention.22
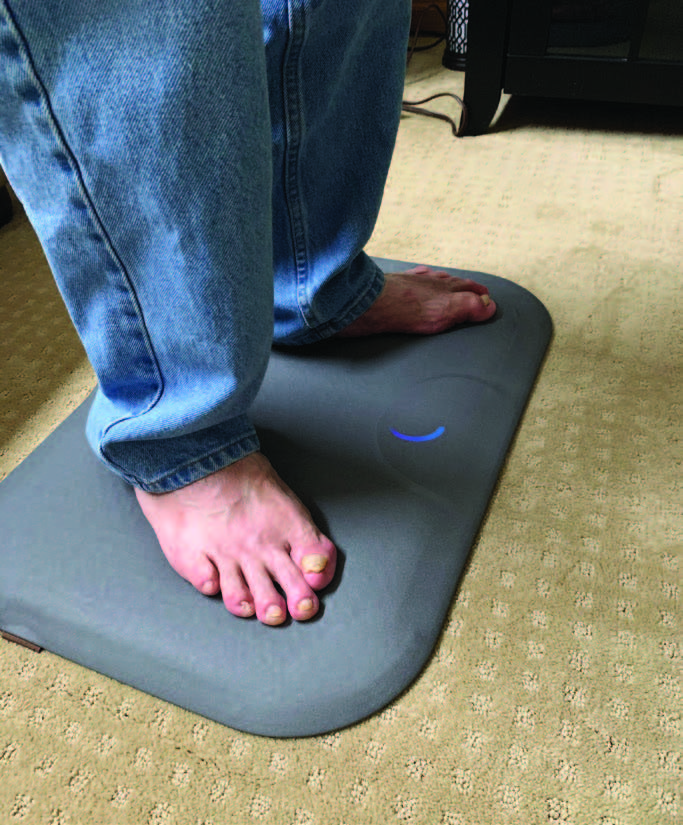
The Podimetrics remote temperature monitoring system (Podimetrics, Inc.) is another example of how technology is changing the way we treat high-risk patients with diabetes by increasing patient engagement, awareness and adherence to a recommended treatment plan. The system utilizes a wireless floor mat with temperature sensors under a water-resistant cover to record a thermogram (temperature scan) of both feet and detect temperature asymmetry, which can be predictive of skin ulceration.
In a multicenter trial evaluating 129 participants with diabetes and a history of DFU over the course of 34 weeks, the Podimetrics system detected 97 percent of observed DFUs nearly six weeks before the clinical appearance of an ulcer.23 Of note, 86 percent of the study cohort used the system at least three days per week on average during the course of the study.
In Conclusion
Managing patients with diabetes can be challenging but can provide true gratification when, in spite of all the confounding variables, one achieves favorable outcomes. In order to heal wounds and, more importantly, keep those wounds healed, providers and their teams should view patients as essential partners in the quest for limb preservation.
Dr. Isaac is the Director of Research at Foot and Ankle Specialists of the Mid-Atlantic (FASMA) in Rockville, Maryland. He is a Fellow of the American College of Foot and Ankle Surgeons (ACFAS).
1. International Diabetes Federation. IDF Diabetes Atlas, 9th ed. Brussels, Belgium, 2019. Available at: http://www.diabetesatlas.org. Accessed May 6, 2020.
2. Centers for Disease Control and Prevention. National diabetes statistics report 2017. Available at: https://dev.diabetes.org/ sites/default/files/2019-06/cdc-statistics-report-2017.pdf . Accessed May 6, 2020.
3. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217-228.
4. Chatterjee JS. From compliance to concordance in diabetes. J Med Ethics. 2006;32(9):507-510.
5. World Health Organisation. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organisation; 2003. Available at: https://www.who. int/chp/knowledge/publications/adherence_report/en/ . Accessed May 15, 2020.
6. Price P. How can we improve adherence? Diab Metab Res Rev. 2016;32(S1):201-205.
7. International Working Group on the Diabetic Foot. Guidelines. Available at: https:// iwgdfguidelines.org/guidelines/guidelines/ . Accessed May 1, 2020.
8. Wounds International. Best practice guidelines: wound management in diabetic foot ulcers. Available at: https://www.woundsinternational.com/resources/details/best-practice-guidelines-wound-management-diabetic-foot-ulcers . Published May 10, 2013. Accessed May 6, 2020.
9. Devine F, Edwards T, Feldman SR. Barriers to treatment: describing them from a different perspective. Patient Prefer Adherence. 2018;12:129-133.
10. Armstrong DG, Lavery LA, Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366(9498):1704- 1710.
11. Haugh AM, Witt JG, Hauch A, et al. Amnion membrane in diabetic foot wounds: a meta-analysis. Plastic Recon Surg Glob Open. 2017;5(4):e1302.
12. Brand PW. Diabetic foot. In: Ellenberg M, Rifkin H, eds. Diabetes Mellitus: Theory and Practice, 3rd ed. New York: Medical Examination Publishing, 1983, pp. 829-49.
13. Cavanagh PR, Bus SA. Off-loading the diabetic foot for ulcer prevention and healing. Plastic Recon Surg. 2011;127(Suppl 1):248S- 256S.
14. Bus SA, Van Deursen RW, Armstrong DG, Lewis JE, Caravaggi CF, Cavanagh PR, International Working Group on the Diabetic Foot (IWGDF). Footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in patients with diabetes: a systematic review. Diabetes Metab Res Rev. 2016;32(Suppl 1):99-118.
15. Wu SC, Jensen JL, Weber AK, Robinson DE, Armstrong DG. Use of pressure offloading devices in diabetic foot ulcers: do we practice what we preach? Diabetes Care. 2008;31(11):2118-2119.
16. Armstrong DG, Isaac AL, Bevilacqua NJ, Wu SC. Offloading foot wounds in people with diabetes. Wounds. 2014;26(1):13-20.
17. Lavery LA, Vela SA, Lavery DC, Quebedeaux TL. Reducing dynamic foot pressures in high-risk diabetic subjects with foot ulcerations: a comparison of treatments. Diabetes Care. 1996;19(8):818-821.
18. Armstrong DG, Lavery LA, Kimbriel HR, Nixon BP, Boulton AJ. Activity patterns of patients with diabetic foot ulceration: patients with active ulceration may not adhere to a standard pressure off-loading regimen. Diabetes Care. 2003;26(9):2595-2597.
19. Katz IA, Harlan A, Miranda-Palma B, et al. A randomized trial of two irremovable off-loading devices in the management of plantar neuropathic diabetic foot ulcers. Diabetes Care. 2005;28(3):555-559.
20. Piaggesi A, Macchiarini S, Rizzo L, et al. An off-the-shelf instant contact casting device for the management of diabetic foot ulcers: a randomized prospective trial versus traditional fiberglass cast. Diabetes Care. 2007;30(3):586-590.
21. Piaggesi A, Goretti C, Iacopi E, et al. Comparison of removable and irremovable walking boot to total contact casting in offloading the neuropathic diabetic foot ulceration. Foot Ankle Int. 2016;37(8):855- 861.
22. Sensoria Health. Diabetes and foot complications. Available at: https://www.sensoriahealth.com/motus-smart/ . Accessed May 6, 2020.
23. Frykberg RG, Gordon IL, Reyzelman AM, et al. Feasibility and efficacy of a smart mat technology to predict development of diabetic plantar ulcers. Diabetes Care. 2017;40(7):973-980.