Cerebrovascular Events With Self-Expanding Versus Balloon-Expandable Valves in Patients With or Without Peripheral Arterial Disease
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
J INVASIVE CARDIOL 2025. doi:10.25270/jic/25.00020. Epub February 21, 2025.
Abstract
Objectives. The authors compared the risk of cerebrovascular events (CVE) with self-expanding vales (SEV) vs balloon-expandable valves (BEV) in patients with or without peripheral artery disease (PAD), stratified by the access route and the complexity of PAD (Hostile score).
Methods. The PAD-related risk of CVE between SEV vs BEV was investigated using data from the HOSTILE Registry, an observational study including 1707 patients with severe PAD undergoing transcatheter aortic valve replacement (TAVR) via different access routes. The relative risk of CVE with SEV vs BEV in patients without PAD was investigated in a meta-analysis of randomized controlled transfemoral access (TFA)-TAVR trials of patients with normal femoral arteries. The primary endpoint was the risk of 30-day CVE.
Results. Among the 1021 patients undergoing TAVR through TFA or transaxillary access (TAxA), 674 (66.0%) received SEVs and 329 (32.2%) received BEVs. The 30-day propensity-adjusted risk of CVE was higher for SEV compared with BEV (adjusted hazard ratio [HR], 2.70; 95% CI, 1.16-6.23), with no significant interaction between the transcatheter heart valve and either the access route or the Hostile score. Similar results were apparent at 1 year (adjusted HR, 2.98; 95% CI, 1.30-6.83). In contrast, in a meta-analysis of 4 RCTs and 2131 patients with femoral arteries suitable for TAVR, there were no significant differences in the 30-day rates of CVE between SEV and BEV (odds ratio, 0.58; 95% CI, 0.24-1.40).
Conclusions. Compared with BEVs, SEVs were associated with higher 30-day and 1-year rates of CVE in patients with PAD, a finding not apparent in patients with suitable femoral arteries enrolled in RCTs.
Introduction
Cerebrovascular events (CVE) after transcatheter aortic valve replacement (TAVR) are a serious complication associated with substantial morbidity and mortality.1 Risk predictors of CVE in patients undergoing TAVR are poorly understood and, to our knowledge, have not been investigated in patients with concomitant peripheral arterial disease (PAD).2 Identifying patients at higher risk of CVE after TAVR would inform selection of the patients most likely to benefit from embolic protection devices.3
We recently reported the results of the HOSTILE Registry, a large-scale, international, multicenter observational registry of patients with severe PAD undergoing TAVR via transfemoral access (TFA) following percutaneous treatment of PAD; transthoracic access (TTA), including transapical and transaortic approaches; or non-femoral, non-thoracic alternative access, including transaxillary, transcarotid, transbrachicephalic, and transcaval access routes.4 In that study,4 a novel score (Hostile score) was generated to characterize the extent and severity of PAD. The score contains 7 anatomic variables, including the number of iliofemoral segments with significant disease, the presence of obstruction in any segment, iliac disease involving the aortic bifurcation, tortuosity, calcification, lesion length, and the minimal lumen diameter. Of note, TFA-treated patients with high Hostile scores had higher rates of CVE compared with those with low Hostile scores, which is likely related to greater atherosclerotic disease in the aortic arch and ascending aorta.4 In addition, patients undergoing TAVR via transaxillary access (TAxA) had increased rates of CVE compared with TFA or TTA patients. Other studies have reported similar findings, but none has investigated whether CVE rates vary according to the type of transcatheter heart valves (THV) and severity of PAD.5
Therefore, we analyzed the risk of CVE with self-expanding valves (SEV) vs balloon-expandable valves (BEV) in patients enrolled in the HOSTILE Registry and treated via TFA or TAxA, stratifying patients by access route and the Hostile score. To investigate the relative risk of CVE with SEV vs BEV in patients with femoral arteries without severe PAD suitable for the transfemoral approach, a parallel pairwise aggregate data-based meta-analysis of randomized controlled trials (RCT) was performed.
Methods
Study design and objectives
The design of the HOSTILE Registry has already been reported.4 Briefly, HOSTILE was a multicenter, international study including retrospective and prospective data collection in patients with severe PAD undergoing TAVR. This “all-comers” registry included consecutive patients undergoing TAVR with “hostile femoral access”, defined as extensive bilateral iliofemoral PAD so severe as to require either PAD intervention prior to femoral access to enable TAVR through the femoral approach, or requiring TAVR via alternate (non-femoral) access. For the purpose of the present study, we selected only patients treated with SEV or BEV through TFA or TAxA. The principal objective was to compare 30-day and 1-year clinical outcomes between SEV vs BEV. The secondary objective was to analyze results in patients stratified by the access route and by the Hostile score, as previously described.4 All patients were censored at 1 year. The authors confirm that the study has adhered to the Declaration of Helsinki, and informed consent was obtained from the patients for the study described in the manuscript and for the publication thereof.
Finally, to appraise the relative risk of CVE with SEV vs BEV in patients with no PAD, we performed in parallel an aggregate data-based meta-analysis of RCTs enrolling patients undergoing TAVR with SEV or BEV and who had femoral arteries without severe PAD suitable for the transfemoral approach. The search strategy and methods of the meta-analysis are reported in Appendix A.
Endpoints and definitions
The primary endpoint of the study was the propensity-adjusted 30-day risk of CVE, defined as the composite of stroke or transient ischemic attack (TIA). Secondary endpoints were the primary endpoint at 1 year, as well as all-cause mortality, cardiac mortality, stroke, myocardial infarction, Valve Academic Research Consortium (VARC)-3 major vascular complications, and VARC-3 major bleeding complications at 30 days and 1 year. Combinations of these endpoints at 30 days and 1 year were also determined. All clinical endpoints were defined according to the VARC-3 definitions.6 For the meta-analysis, the primary endpoint was the 30-day risk of CVE with SEV vs BEV.
Statistical analysis
Continuous variables are reported as means with SD and were compared using the Student t-test. Categorical variables are reported as counts and percentages and were compared using the χ2 statistic. Survival analyses were performed using the Kaplan-Meier method and differences between groups were analyzed with the log-rank test. To adjust for possible confounders, Cox regression multivariable analyses were performed, adjusting for the propensity score of patients receiving SEV. The propensity score contained both clinical and anatomic variables, as specified in Supplemental Table 1. To assess the overall balance of covariates included in the propensity score, we calculated the summary measure by taking the maximum of the standardized mean differences for each treatment. Interaction analyses were performed between the type of THV (SEV or BEV) and either the access route (TFA vs TAxA) or the Hostile score (≤ 8.5 vs > 8.5). For the latter analyses, adjustments were performed using a propensity score containing clinical variables only (Supplemental Table 1).
The statistical methods for the meta-analysis are reported in Appendix A. Two-sided P-values of less than 0.05 were considered statistically significant. Statistical analyses were performed using Stata/IC 16.1 for Windows (StataCorp) and R version 4.0.3 (https://www.r-project.org).
Results
Among the 1707 patients enrolled in the HOSTILE Registry, 1021 were treated through either TFA or TAxA. Among these, 674 (66.0%) received SEVs and 329 (32.2%) received BEVs; in 18 (1.8%) patients, the type of THF was not specified. The clinical and procedural characteristics of patients stratified by THV are reported in Tables 1 and 2. TAxA was used more frequently for SEVs, while TFA was used more frequently for BEVs. The overall balance of covariates included in the propensity score is reported in Supplemental Table 2.


HOSTILE Registry analysis: 30-day outcomes
The clinical outcomes at 30 days are reported in Table 3, Figure 1, and Supplemental Table 3. CVE rates at 30 days were 5.2% in SEV patients and 2.2% in BEV patients (P = .03). After propensity score adjustment, SEV was associated with higher 30-day rates of CVE compared with BEV (adjusted hazard ratio [HR], 2.70; 95% CI, 1.16-6.23), mainly driven by increased rates of stroke (4.0% vs 1.6%, respectively; adjusted HR, 2.58; 95% CI, 1.00-6.86). Rates of CVE stratified by the access route and the Hostile score are shown in Figure 2. Among patients treated through TFA, CVE rates were 3.3% with SEV vs 1.8% with BEV (adjusted HR, 2.15; 95% CI, 0.66-7.05); among those treated through TAxA, the CVE rates were 6.8% with SEV vs 3.0% with BEV (adjusted HR, 2.76; 95% CI, 0.78-9.78; P = .78). Among patients with a low Hostile score, CVE rates were 4.4% with SEV and 2.5% with BEV (adjusted HR, 2.62; 95% CI, 0.93-7.33); among those with a high Hostile score, the CVE rates were 6.5% with SEV vs 1.7% with BEV (adjusted HR, 4.49; 95% CI, 1.03-19.61; P= .56). When the analysis was restricted to TFA patients only (Figure 3), rates of CVE with SEV vs BEV trended higher in patients with a high Hostile score (8.6% with SEV vs 1.6% with BEV; adjusted HR, 6.90; 95% CI, 0.80-59.71, P = .07); the rates were similar in patients with a low Hostile score (1.8% with SEV vs 1.9% with BEV; adjusted HR, 1.13; 95% CI, 0.25-5.06; P = .18). Composite outcomes at 30 days are reported in Table 3 and Supplemental Figure 1.






Figure 1. Kaplan-Meier survival curves for the 30-day risks of clinical outcomes stratified by the transcatheter heart valve. (A) Cerebrovascular events including the composite of stroke and transient ischemic attack; (B) stroke, (C) all-cause mortality, (D) cardiac mortality, and (E) main access related VARC-3 major vascular complications. BEV = balloon-expandable valves; HR = hazard ratio; SEV = self-expanding valves; VARC = Valve Academic Research Consortium.

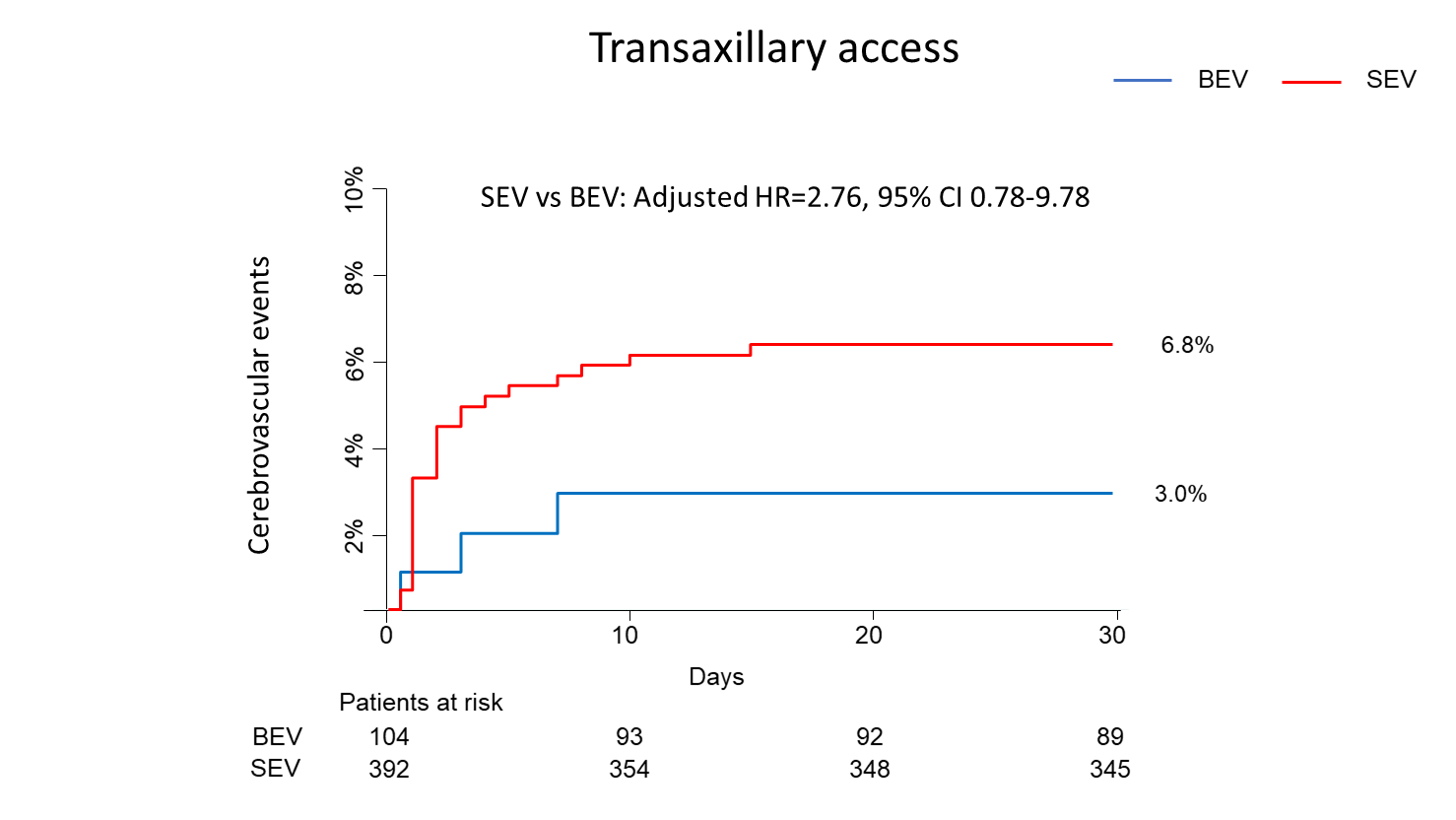


Figure 2. Kaplan-Meier survival curves for the 30-day risk of cerebrovascular events with self-expanding vs balloon-expandable valves stratified by the access route and by the Hostile score: (A) transfemoral access, (B) transaxillary access, (C) low Hostile score, (D) high Hostile score. BEV = balloon-expandable valves; HR = hazard ratio; SEV = self-expanding valves.


Figure 3. Kaplan-Meier survival curves for the 30-day risk of cerebrovascular events with self-expanding vs balloon-expandable valves in transfemoral-treated patients stratified by the Hostile scores: (A) high Hostile score, (B) low Hostile score. BEV = balloon-expandable valves; SEV = self-expanding valves.
HOSTILE Registry analysis: 1-year outcomes
Clinical outcomes at 1 year are reported in Table 4, Figure 4, and Supplemental Table 4. CVE at 1-year occurred in 6.0% of SEV patients and 2.2% of BEV patients (P = .03). After propensity score adjustment, SEV was associated with higher 1-year rates of CVE compared with BEV (adjusted HR, 2.98; 95% CI, 1.30-6.83), mainly driven by increased rates of stroke (4.6% vs 1.6%, respectively; adjusted HR, 2.84; 95% CI, 1.08-7.47). Composite outcomes at 1 year are reported in Table 4 and Supplemental Figure 2.





Figure 4. Kaplan-Meier survival curves for the 1-year risks of clinical outcomes stratified by the transcatheter heart valve: (A) cerebrovascular events including the composite of stroke and transient ischemic attack, (B) stroke, (C) all-cause mortality, and (D) cardiac mortality. BEV = balloon-expandable valves; HR = hazard ratio; SEV = self-expanding valves.
Pairwise meta-analysis
Four RCTs in which severe PAD was absent were identified for the meta-analysis for a total of 2131 patients, 1063 of whom were treated with SEV and 1068 with BEV (Supplemental Figure 3).7-9 Characteristics of the studies and the patients included in the meta-analysis are reported in Supplemental Tables 5 and 6. Among these patients, there were no significant differences in the 30-day rates of CVE between SEV and BEV (OR, 0.58; 95% CI, 0.24-1.40) (Figure 5). Risk of bias is reported in Supplemental Figure 4. The PRISMA checklist is reported in Appendix B.

Discussion
To our knowledge, this is the first study to investiage the risk of CVE with SEV vs BEV in patients with or without PAD treated via either TFA or TAxA. The major findings of this study are as follows: (1) in patients with PAD, SEV was associated with higher 30-day and 1-year rates of CVE compared with BEV, mainly driven by higher rates of stroke; (2) there were no significant interactions between THV and access route, nor between THV and the Hostile score for the risk of CVE; and, (3) in contrast, in patients without severe PAD, an aggregate data-based meta-analysis suggested no significant difference in the 30-day rates of CVE between SEV and BEV.
In the HOSTILE Registry of patients with severe PAD undergoing TAVR for severe aortic valve stenosis, patients treated by TFA or TAxA had significantly lower 30-day rates of major adverse cardiovascular events compared with patients treated through the TTA, including transapical and transaortic access.4 Moreover, patients treated with TAxA and those treated with TFA with high Hostile scores had higher rates of CVE than those treated with TFA with low Hostile scores.
The predictors of CVE after TAVR are poorly understood. In this regard, the relative risk of CVE with SEV vs BEV is uncertain. Randomized controlled trials comparing SEV vs BEV in patients without severe PAD who were suitable for TFA were underpowered for the risk of CVE,7-9 and no prior study has investigated the relative CVE risk of SEV vs BEV in patients with severe PAD. We therefore investigated CVE differences with SEV vs BEV across the entire spectrum of TAVR patients, including patients with severe PAD enrolled in the HOSTILE Registry and patients without severe PAD enrolled in RCTs comparing SEV vs BEV after TFA.7-9 For the HOSTILE Registry analysis, we included both TFA and TAxA-treated patients because these were the categories of patients at the highest risk of CVE. Different results were apparent in the HOSTILE Registry analysis vs the meta-analysis of RCTs. Specifically, in patients with severe PAD enrolled in the registry, SEVs were associated with higher 30-day and 1-year rates of CVE compared with BEVs, with no significant interaction between THV and either the access route (TFA or TAxA) or the Hostile score, whereas, in patients without PAD enrolled in RCTs, no significant difference was apparent between SEVs and BEVs.
These data suggest that the relative risk of CVE with SEV vs BEV may be modulated by the presence of PAD and therefore related to different mechanisms. PAD is often associated with polyvascular disease that also involves the aortic arch and ascending aorta from which cerebral emboli may arise.10 In contrast to BEV, SEV systems cannot be flexed when crossing the aortic arch, and may therefore be more likely to interact with friable atherosclerotic debris attached to the vessel wall, causing cerebral embolism. In addition, unsheathed valves may be more likely to scrape iliofemoral atherosclerotic debris with the nose of the delivery system producing retrograde embolization at the level of the epiaortic vessels. Consistent with this hypothesis, we found that SEVs were associated with 7-fold higher rates of CVE compared with BEV in TFA patients with high Hostile scores, but similar CVE rates as BEV in TFA patients with low Hostile scores. Conversely, in patients without severe PAD enrolled in RCTs in whom atherosclerotic disease affecting the aortic arch and ascending aorta is likely to be less, no significant difference was apparent between the 2 types of THV.
In the HOSTILE Registry, patients undergoing TAVR via TAxA had higher rates of CVE compared with patients treated via TFA or TTA.4 Of note, in the present analysis there was no interaction between THV and access route; CVE rates were still more than twice as high with SEV compared with BEV (6.8% vs 3.0%) in patients treated via TAxA. Access-site specific mechanisms, in addition to polyvascular disease, may thus play a role. Specifically, the vertebrobasilar vascular system supplying the posterior part of the brain raises from the posterosuperior aspect of the central subclavian artery, which is more fragile and more prone to dissection than the femoral artery. It is therefore possible that SEV, which cannot be flexed to negotiate tortuous axillary and subclavian arteries and aortic arch, may more frequently cause vascular dissections and embolization of atherosclerotic debris with ischemia of the vertebrobasilar vascular system.
New and more flexible delivery systems have recently been introduced to mitigate this risk. Specifically, the Evolut FX system (Medtronic) has been engineered with only 1 spine and with a redesigned tip and a more flexible capsule.11 These modifications may confer greater flexibility and trackability to the valve, allowing it to more readily negotiate complex anatomies, avoid excessive friction against the aortic arch, and better navigate through the axillary vascular system. Whether these features will reduce CVE risk and improve clinical outcomes in patients with PAD deserves further investigation.
The results of the present study in patients with severe PAD were based on the observational HOSTILE Registry and should not be equated to findings of RCTs. Although we adjusted for important covariates, unmeasured confounders may remain. Because the study was not randomized, the results from the present study should be considered hypothesis-generating. Clinical events were not centrally adjudicated. The use of cerebral embolic protection devices was not captured in this registry, although studies to date have not shown reduction in clinical stroke rates with these devices.3 The meta-analysis included only 4 randomized trials; more randomized data comparing outcomes of different valve types in patients with and without PAD are needed.
Conclusions
In the present study from the HOSTILE Registry, among patients with severe PAD undergoing TAVR through TFA or TAxA, SEVs were associated with higher 30-day and 1-year rates of CVE compared with BEVs, with no significant interaction between THV and either the access route or the complexity and severity of PAD. In patients with severe PAD undergoing TAVR by TFA after percutaneous treatment, CVE rates trended higher with SEV vs BEV only in those with high Hostile scores. Finally, from RCTs in patients without severe PAD who were suitable for TFA, no significant difference in the 30-day rates of CVE was apparent between SEVs and BEVs, suggesting that the valve types are associated with differing risks of CVE depending on the severity of PAD.
Affiliations and Disclosures
Tullio Palmerini, MD1,2; Francesco Saia, MD, PhD1,2; Antonio Giulio Bruno, MD1,2; Won-Keun Kim, MD3; Alessandro Iadanza, MD4; Gabriele Ghetti, MD1,2; Ole De Backer, MD5; Francesco Burzotta, MD6; Nicolas M. Van Mieghem, MD7; Elena Nardi, Mstat8; Mateusz Orzalkiewicz, MD1,2; Thomas Pilgrim, MD, MSc9, Tiziana Claudia Aranzulla, MD, MSc10; Max M. Meertens, MD11; Nevio Taglieri, MD1,2; Michael Joner, MD12, Giulia Nardi, MD13,14; Stefan Toggweiler, MD14; Enrico Gallitto, MD15; Mauro Gargiulo, MD15; Luca Testa, MD16; Sergio Berti, MD17; Matteo Montorfano, MD18; Alessandro Leone, MD19; Davide Pacini, MD19; Daniel Braun, MD20; Fausto Castriota, MD21; Marco De Carlo, MD22; Marco Barbanti, MD23; Pier Pasquale Leone, MD24; Georg Nickenig, MD25; Tommaso Piva, MD26; Azeem Latib, MD27,28; Matteo Vercellino, MD29; Pablo Codner, MD30; Antonio L. Bartorelli, MD31; Chiara Fraccaro, MD32; Mohamed Abdel-Wahab, MD33; Gregg W. Stone, MD34
From the 1Cardiology Unit, Cardiac Thoracic and Vascular Department, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; 2Cardiac Thoracic and Vascular Department, Università di Bologna, Bologna, Italy; 3Kerckhoff Heart Center, Bad Nauheim, Germany; 4UOSA Cardiologia Interventistica Azienda Ospedaliera Universitaria Senese, Siena, Italy; 5The Heart Center, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark; 6U.O.C. di Interventistica Cardiologica e Diagnostica Invasiva, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy; 7Department of Cardiology, Thoraxcenter, Erasmus University Medical Center, Rotterdam, the Netherlands; 8Research and Innovation Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; 9Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland; 10A.O. Mauriziano Umberto I Hospital, Turin, Italy; 11University Hospital Cologne – Heart Center, Klinik III für Innere Medizin - Kardiologie, Pneumologie und internistische Intensivmedizin, Cologne, Germany; 12German Heart Centre Munich/Deutsches Herzzentrzum München - Munich, Germany; 13University Hospital Careggi, Florence, Italy; 14Heart Center Lucerne, Cardiology, Luzerner, Switzerland; 15Vascular Surgery, University of Bologna, DIMEC, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; 16Coronary Revascularisation Unit, IRCCS Policlinico S. Donato, San Donato Milanese, Italy; 17Unit of Diagnostic and Interventional Cardiology, C.N.R. Reg. Toscana G. Monasterio Foundation, Ospedale del Cuore, Massa, Italy; 18Interventional Cardiology Unit - IRCCS San Raffaele Scientific Institute, Milan, Italy; 19Cardiac Surgery Unit, Cardiac Thoracic and Vascular Department, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Coronary Revascularisation Unit, IRCCS Policlinico S. Donato, S. Donato Milanese, Italy; 20Department of Medicine I, University Hospital Munich, Medical faculty - LMU, Munich, Germany; 21Interventional Cardiology Unit - GVM Care and Research Maria Cecilia Hospital - Cotignola (RA), Italy; 22Cardiothoracic and Vascular Department, Pisa University Hospital, Pisa, Italy; 23Division of Cardiology, Policlinico-Vittorio Emanuele Hospital, University of Catania, Catania, Italy; 24Cardio Center, Humanitas Research Hospital IRCCS, Rozzano-Milan, Italy; 25Medizinische Klinik und Poliklinik II, Herzzentrum Bonn - Universitätsklinikum Bonn, Bonn, Germany; 26Azienda Ospedaliero-Universitaria, Ospedali Riuniti Umberto I - GM Lancisi, Torette, Ancona, Italy; 27Montefiore-Einstein Center for Heart and Vascular Care, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, New York; 28Cardiovascular Research Foundation, New York, New York; 29Cardiology Unit, Cardio-Thoraco Vascular Department (DICATOV), IRCCS Ospedale Policlinico San Martino, Genoa, Italy; 30Rabin Medical Center, Petah Tikva, Israel; 31Unità Operativa di Cardiologia Interventistica Universitaria, IRCCS Ospedale Galeazzi - Sant’Ambrogio, Milano Milan, Italy; 32Department of Cardiac, Thoracic and Vascular Sciences, University of Padua, Padua, Italy; 33Heart Center Leipzig at University of Leipzig, Leipzig, Germany; 34Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, New York.
Acknowledgments: The authors thank the following investigators for their invaluable contribution to the manuscript: Matthias Renker, MD (Kerckhoff Heart Center, Bad Nauheim, Germany); Massimo Fineschi, MD (UOSA Cardiologia Interventistica Azienda Ospedaliera Universitaria Senese, Siena, Italy); Alessandro Mazzapicchi, MD (Cardiology Unit, Cardiac Thoracic and Vascular Department, IRCCS Azienda Ospedaliero-Universitaria di Bologna, and Cardiac Thoracic and Vascular Department, Università di Bologna, Italy); Maarten Vanhaverbeke, MD (The Heart Center, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark); Enrico Romagnoli, MD (U.O.C. di Interventistica Cardiologica e Diagnostica Invasiva, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy); Carlo Trani, MD (U.O.C. di Interventistica Cardiologica e Diagnostica Invasiva, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy); Rik Adrichem, MD (Department of Cardiology, Thoraxcenter, Erasmus University Medical Center, Netherlands); Daijiro Tomii, MD (Inselspital, Bern University Hospital, University of Bern, Switzerland); Giuseppe Musumeci, MD (A.O. Mauriziano Umberto I Hospital, Turin, Italy); Matti Adam, MD (University Hospital Cologne – Heart Center, Klinik III für Innere Medizin - Kardiologie, Pneumologie und internistische Intensivmedizin, Cologne, Germany); Hector Alfonso Alvarez Covarrubias, MD (German Heart Centre Munich/Deutsches Herzzentrzum München - Munich, Germany, and Hospital de Cardiología, Centro Médico Nacional Siglo XXI, IMSS, Ciudad de México); Francesco Meucci, MD (University Hospital Careggi, Florence, Italy); Carlo Di Mario, MD (University Hospital Careggi, Florence, Italy); Lucca Loretz, MD (Heart Center Lucerne, Cardiology, Luzerner, Switzerland); Francesco Bedogni, MD (Coronary Revascularisation Unit, IRCCS Policlinico S. Donato, S. Donato Milanese, Italy); Marco B. Ancona, MD (Interventional Cardiology Unit - IRCCS San Raffaele Scientific Institute, Milan, Italy); Jonas Gmeiner, MD (Department of Medicine I, University Hospital Munich, Medical faculty - LMU, Munich, Germany); Roberto Nerla, MD (Interventional Cardiology Unit - GVM Care and Research Maria Cecilia Hospital - Cotignola (RA), Italy); Pier Pasquale Leone, MD (Cardio Center, Humanitas Research Hospital IRCCS, Rozzano-Milan, Italy); Giulio Stefanini, MD (Cardio Center, Humanitas Research Hospital IRCCS, Rozzano-Milan, Italy); Mitsumasa Sudo, MD (Medizinische Klinik und Poliklinik II, Herzzentrum Bonn - Universitätsklinikum Bonn, Bonn, Germany); Andrea Scotti, MD (Montefiore-Einstein Center for Heart and Vascular Care, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, New York); Italo Porto, MD (Cardiology Unit, Cardio-Thoraco Vascular Department (DICATOV), IRCCS Ospedale Policlinico San Martino, Genoa, Italy); Ran Kornowski, MD (Rabin Medical Center, Petah Tikva, Israel); Giuseppe Tarantini, MD (Department of Cardiac, Thoracic and Vascular Sciences, University of Padua, Padua, Italy); Nazzareno Galié, MD (Cardiology Unit, Cardiac Thoracic and Vascular Department, IRCCS Azienda Ospedaliero-Universitaria di Bologna; Cardiac Thoracic and Vascular Department, Università di Bologna, Italy).
Disclosures: Dr Palmerini has received consultancy and lecture fees from Edwards Lifesciences, Medtronic, and Abbott. Dr Saia has received consultancy and lecture fees from Abbott, Edwards Lifesciences, and Medtronic, and is a proctor for Medtronic. Dr Kim has received personal fees from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, Meril Life Sciences, and Shockwave Medical. Dr Burzotta has received speaker’s fees from Abiomed, Abbott, Medtronic, and Terumo. Dr Van Mieghem has received research grant support from Abbott Vascular, Biotronik, Boston Scientific, Medtronic, Edwards Lifesciences, Abiomed, PulseCath BV, and Daiichi Sankyo. Dr Pilgrim reports research, travel, or educational grants to the institution without personal remuneration from Biotronik, Boston Scientific, Edwards Lifesciences, and ATSens; and receives speaker fees and consultancy fees to the institution from Biotronik, Boston Scientific, Edwards Lifesciences, Abbott, Medtronic, Biosensors, and Highlife. Dr Toggweiler is a proctor/consultant for Medtronic, Edwards Lifesciences, Biosensors, Boston Scientific, and Abbott Vascular; a consultant for Medira, AtHeart Medical, Veosource, Shockwave, Teleflex, and Polares Medical; has received institutional research grants from Biosensors, Boston Scientific, and Fumedica; and holds equity in Hi-D Imaging. Dr Testa has received consulting fees from Abbott, Boston, Medtronic, and Meril, and has been proctor for Abbott, Boston, Medtronic, and Meril. Dr Berti is a proctor for Edwards Lifesciences, Abbott, and Boston Scientific. Dr Montorfano reports proctor fees from Abbott, Edwards Lifesciences, and Boston Scientific. Dr Castriota reports proctoring fees from Abbott and Medtronic. Dr Barbanti reports to be a consultant for Medtronic, Edwards Lifesciences, and Boston Scientific. Dr Nickenig reports honoraria for lectures or advisory boards from Abbott, Amarin, AstraZeneca, Bayer, Berlin Chemie, Biosensus, Biotronic, BMS, Boehringer Ingelheim, Cardiovalve, Daiichi Sankyo, Edwards, Medtronic, Novartis, Pfizer, and Sanofi Aventis; stock options with Beren and Cardiovalve; participation in clinical trials with Abbott, AstraZeneca, Bayer, Berlin Chemie, Biosensus, Biotronic, BMS, Boehringer Ingelheim, Cardiovalve, Daiichi Sankyo, Edwards, Medtronic, Novartis, Pfizer, and Sanofi Aventis; and research funding from DFG, BMBF, EU, Abbott, Bayer, BMS, Boehringer Ingelheim, Edwards Lifesciences, Medtronic, Novartis, and Pfizer. Dr Latib has served as a consultant for Abbott, Medtronic, Edwards Lifesciences, Boston Scientific, Neovasc, Shifamed, and Philips. Dr Stone has received speaker honoraria from Medtronic, Pulnovo, Abiomed, Amgen, and Boehringer Ingelheim; has served as a consultant to Abbott, Daiichi Sankyo, Ablative Solutions, CorFlow, Cardiomech, Robocath, Miracor, Vectorious, Apollo Therapeutics, Elucid Bio, Cardiac Success, Valfix, TherOx, HeartFlow, Neovasc, Ancora, Occlutech, Impulse Dynamics, Adona Medical, Millennia Biopharma, Oxitope, HighLife, Elixir, Remote Cardiac Enablement, and Aria; and has equity/options in Cardiac Success, Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Valfix, and Xenter. In addition, Dr Stone’s employer, Mount Sinai Hospital, receives research grants from Shockwave, Abbott, Abiomed, Bioventrix, Cardiovascular Systems Inc, Phillips, Biosense-Webster, Vascular Dynamics, Pulnovo, and V-Wave. The remaining authors report no financial relationships or conflicts of interest regarding the content herein.
Address for correspondence: Tullio Palmerini, MD, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Via Massarenti 9, Bologna 40138, Italy. Email: tulliopalmerini@hotmail.com; X: @tulliopalmerini
Supplemental Material
APPENDIX A: METHODS FOR THE META-ANALYSIS
Data source and study selection. Relevant randomized controlled trials comparing self-expanding valves (SEV) versus balloon expandable valves (BEV) to include in this meta-analysis were identified through the MEDLINE, Cochrane database, and EMBASE database searches, using the keywords coronary (((transcatheter aortic valve replacement) OR (transcatheter aortic valve implantation)) OR (self-expanding valve)) OR (balloon-expandable valve)) OR (Sapien)) OR (evolute)) OR (COREVALVE)) OR (TAVI)) OR (TAVR). We did not limit the search to English language. We considered only trials enrolling patients with femoral arteries suitable for the transfemoral approach. Trials comparing SEV versus SEV, BEV vs. BEV or with a mixture of SEV and BEV in the treatment or control groups were excluded. The most updated and inclusive data for a given study were chosen for abstraction. Two investigators (TP, AGB) independently reviewed the titles, abstracts and studies to determine whether they met the inclusion criteria. Conflict between reviewers were resolved by consensus.
Statistical analysis. All analyses followed the intention-to-treat principle. Odds ratio (OR) and 95% confidence interval (CI) were used as the summary statistic. The pooled OR was calculated by using both fixed effect (inverse variance weighted) and random effect (DerSimonian and Laird) models. Between-study heterogeneity of effects was analyzed by using the chi square and I2statistic.
APPENDIX B: PRISMA CHECKLIST












Supplemental Figure 1. Kaplan-Meier survival curves for the 30-day risks of composite clinical outcomes stratified by the transcatheter heart valve.
(A) All-cause mortality and cerebrovascular events (CVE); (B) all-cause mortality, CVE or myocardial infarction (MI); (C) all-cause mortality, CVE or VARC-3 major bleeding. Adjusted hazard ratios were calculated using Cox multivariate analyses (see Methods). There were no significant differences in any composite clinical outcome between self-expanding valves (SEV) vs balloon-expandable valves (BEV).



Supplemental Figure 2. Kaplan-Meier survival curves for the 1-year risks of composite clinical outcomes stratified by the transcatheter heart valve. (A) All-cause mortality and cerebrovascular events (CVE); (B) all-cause mortality, CVE or myocardial infarction (MI); (C) all-cause mortality, CVE or VARC 3 major bleeding. Adjusted hazard ratios were calculated using Cox multivariate analyses (see Methods). There were no significant differences in any composite clinical outcome between self-expanding valves (SEV) vs balloon-expandable valves (BEV).


References
1. Eggebrecht H, Schmermund A, Voigtländer T, Kahlert P, Erbel R, Mehta RH. Risk of stroke after transcatheter aortic valve implantation (TAVI): a meta-analysis of 10,037 published patients. EuroIntervention. 2012;8(1):129-138. doi:10.4244/EIJV8I1A20
2. Vlastra W, Jimenez-Quevedo P, Tchétché D, et al. Predictors, incidence, and outcomes of patients undergoing transfemoral transcatheter aortic valve implantation complicated by stroke. Circ Cardiovasc Interv. 2019;12(3):e007546. doi:10.1161/CIRCINTERVENTIONS.118.007546
3. Kapadia SR, Makkar R, Leon M, et al; PROTECTED TAVR Investigators. Cerebral embolic protection during transcatheter aortic-valve replacement. N Engl J Med. 2022;387(14):1253-1263. doi:10.1056/NEJMoa2204961
4. Palmerini T, Saia F, Kim WK, et al. Vascular access in patients with peripheral arterial disease undergoing TAVR: the Hostile registry. JACC Cardiovasc Interv. 2023;16(4):396-411. doi:10.1016/j.jcin.2022.12.009
5. Dahle TG, Kaneko T, McCabe JM. Outcomes Following subclavian and axillary artery access for transcatheter aortic valve replacement: Society of the Thoracic Surgeons/American College of Cardiology TVT registry report. JACC Cardiovasc Interv. 2019;12(7):662-669. doi:10.1016/j.jcin.2019.01.219
6. Généreux P, Piazza N, Alu MC, et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol. 2021;77(21):2717-2746. doi:10.1016/j.jacc.2021.02.038
7. Abdel-Wahab M, Mehilli J, Frerker C, et al; CHOICE investigators. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA. 2014;311(15):1503-1514. doi:10.1001/jama.2014.3316
8. Lanz J, Kim WK, Walther T, et al; SCOPE I investigators. Safety and efficacy of a self-expanding versus a balloon-expandable bioprosthesis for transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis: a randomised non-inferiority trial. Lancet. 2019;394(10209):1619-1628. doi:10.1016/S0140-6736(19)32220-2
9. Thiele H, Kurz T, Feistritzer HJ, et al. Comparison of newer generation self-expandable vs. balloon-expandable valves in transcatheter aortic valve implantation: the randomized SOLVE-TAVI trial. Eur Heart J. 2020;41(20):1890-1899. doi:10.1093/eurheartj/ehaa036
10. Aday AW, Matsushita K. Epidemiology of peripheral artery disease and polyvascular disease. Circ Res. 2021;128(12):1818-1832. doi:10.1161/CIRCRESAHA.121.318535
11. Zaid S, Attizzani GF, Krishnamoorthy P, et al. First-in-Human multicenter experience of the newest generation supra-annular self-expanding Evolut FX TAVR system. JACC Cardiovasc Interv. 202316(13):1626-1635. doi:10.1016/j.jcin.2023.05.004















