ADVERTISEMENT
Coronary Imaging and Intervention During Cardiovascular Collapse: Use of the LUCAS Mechanical CPR Device in the Cardiac Catheterization Laboratory
Abstract: The management of cardiac arrest during coronary angiography and intervention presents substantial challenges. Patients presenting with ST-segment elevation myocardial infarction or following resuscitation from cardiac arrest are at greatest risk and may represent a significant portion of patients in some centers. Timely and effective cardiopulmonary resuscitation (CPR), with manual chest compressions is the primary mode of support though novel circulatory assist devices may have some role. To this end, the use of mechanical compression devices provides multiple patient- and provider-level benefits. This series provides a description of the use of the LUCAS mechanical CPR device and examples of coronary imaging and intervention during mechanical CPR.
J INVASIVE CARDIOL 2012;24:79-83
Key words: cardiopulmonary resuscitation, CPR, LUCAS device
__________________________________________
Cardiac arrest during routine coronary angiography and percutaneous coronary intervention (PCI) is uncommon in contemporary cardiac catheterization laboratories (CCL). However, the frequency of cardiac arrest during procedures may increase in busy centers treating acute myocardial infarction and post-cardiac arrest patients. Resuscitation remains a substantial challenge in itself, and can be more limited during coronary angiography or PCI.
A number of clinical studies have documented the high prevalence of acute coronary occlusions in patients resuscitated from out-of-hospital cardiac arrest (OOHCA).1,2 Acute occlusion has been documented in patients initially found to be in ventricular fibrillation as well as other rhythms included in the broad category of pulseless electrical activity (PEA).3 Early angiography and intervention in such patients, when combined with therapeutic hypothermia (32-34°C), has been shown to improve both the likelihood of survival to hospital discharge and survival with normal, intact neurological function.4-6 These two interventions now play a prominent role in the care of resuscitated patients and are recommended in the latest American Heart Association guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care.7
The population at risk for cardiac arrest during catheterization clearly exists. Up to 2% of the overall PCI volume in some regions may involve patients following cardiac arrest.8 Cardiac arrest complicates the hospital course of approximately 5% of patients with acute myocardial infarction.9 Reticence to perform angiography and PCI in such patients is usually the result of unwarranted concern regarding the patient’s anticipated outcome. An additional consideration is the high likelihood of re-arrest during the procedure or undertaking the procedure during ongoing CPR efforts.
The options for emergent circulatory support in the setting of shock or cardiac arrest have significantly expanded in the last decade. Options include intra-aortic balloon pump (IABP) counterpulsation, percutaneous left ventricular assist devices such as Tandem Heart and Impella, and even emergent cardiopulmonary bypass. However, these devices may not be widely available and are difficult to initiate in a pulseless patient. From this practical standpoint, mechanical CPR devices represent a significant advancement in the treatment of cardiac arrest immediately prior to or during cardiac catheterization.
Mechanical CPR (mCPR) devices have been in use for over 30 years and are now widely available. A simulation comparing experienced resuscitators to the LUCAS mCPR device (Physio-Control, Inc) demonstrated more consistent and superior compressions using mechanical CPR.10 Issues related to resuscitator fatigue are also avoided. Nonetheless, the use of mCPR in resuscitation for OOHCA has been studied with conflicting results.11-13 For CCL purposes, these devices provide a benefit in consistent and high-quality CPR as evidenced by the report from Wyss et al.10 In addition, use of mCPR serves multiple practical and safety needs in the CCL. It frees staff to attend to other critical duties, reduces their proximity to the radiation source, and removes them from the imaging field.
Wagner et al have published a series of patients receiving mCPR using the LUCAS device in the catheterization laboratory.14 Their disappointing patient outcomes reflect the reality of treating cardiac arrest patients acutely in the CCL. Nonetheless, all catheterization laboratories that routinely perform primary PCI will treat patients in this condition, and mCPR serves as a valuable tool. The LUCAS device is easy to operate and coronary imaging and intervention can be performed without significant limitations. This report details 4 patients experiencing cardiac arrest in the setting of acute ST-elevation myocardial infarction (STEMI) and 1 during elective PCI, and it provides images and discussion of coronary angiography and PCI during mCPR.
Case Reports
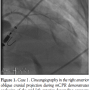 Case 1. A 56-year-old female with no past history and complaining of dyspnea, chest tightness, and profound weakness for 3 hours was brought to the hospital by paramedics. Her pre-hospital electrocardiogram (ECG) demonstrated an acute anterior STEMI. On hospital arrival, she was hemodynamically stable with a blood pressure of 140/67, though she required intubation for respiratory distress. Moments before transport to the catheterization laboratory, she experienced a PEA cardiac arrest requiring CPR and administration of
Case 1. A 56-year-old female with no past history and complaining of dyspnea, chest tightness, and profound weakness for 3 hours was brought to the hospital by paramedics. Her pre-hospital electrocardiogram (ECG) demonstrated an acute anterior STEMI. On hospital arrival, she was hemodynamically stable with a blood pressure of 140/67, though she required intubation for respiratory distress. Moments before transport to the catheterization laboratory, she experienced a PEA cardiac arrest requiring CPR and administration of 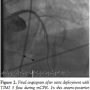 epinephrine and atropine before her pulse was restored. She suffered recurrent cardiac arrest in the CCL and resuscitation efforts were initiated with CPR, epinephrine, and atropine. The LUCAS device was placed and mCPR commenced. An IABP was placed via the right femoral approach and a temporary transvenous pacemaker placed via the right femoral vein. Angiography was performed via left femoral access during mechanical CPR. Her mid LAD was occluded with TIMI 0 flow (Figure 1). Balloon angioplasty was performed promptly with rapid restoration of TIMI 3 flow and placement of a bare-metal stent in the mid LAD (Figure 2). The semi-radiolucent LUCAS device was not visible on coronary angiography or during PCI in a steep right anterior oblique cranial projection. Despite maximal support with an IABP and multiple vasopressors, the patient manifested no significant myocardial function and expired in the catheterization laboratory after mCPR was discontinued.
epinephrine and atropine before her pulse was restored. She suffered recurrent cardiac arrest in the CCL and resuscitation efforts were initiated with CPR, epinephrine, and atropine. The LUCAS device was placed and mCPR commenced. An IABP was placed via the right femoral approach and a temporary transvenous pacemaker placed via the right femoral vein. Angiography was performed via left femoral access during mechanical CPR. Her mid LAD was occluded with TIMI 0 flow (Figure 1). Balloon angioplasty was performed promptly with rapid restoration of TIMI 3 flow and placement of a bare-metal stent in the mid LAD (Figure 2). The semi-radiolucent LUCAS device was not visible on coronary angiography or during PCI in a steep right anterior oblique cranial projection. Despite maximal support with an IABP and multiple vasopressors, the patient manifested no significant myocardial function and expired in the catheterization laboratory after mCPR was discontinued.
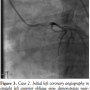 Case 2. A 40-year-old female with a history of hypothyroidism was in a local office when she experienced the sudden onset of crushing chest pain. Emergency medical services (EMS) performed a pre-hospital ECG demonstrating significant ST elevations in leads I, aVL, aVR, and V4-V6. On arrival to the emergency department, she was somnolent with a blood pressure of 114/70 mm Hg and heart rate of 90 bpm. Her ECG evolved into a wide right bundle branch block with anterior STEMI. She was brought to the CCL emergently and
Case 2. A 40-year-old female with a history of hypothyroidism was in a local office when she experienced the sudden onset of crushing chest pain. Emergency medical services (EMS) performed a pre-hospital ECG demonstrating significant ST elevations in leads I, aVL, aVR, and V4-V6. On arrival to the emergency department, she was somnolent with a blood pressure of 114/70 mm Hg and heart rate of 90 bpm. Her ECG evolved into a wide right bundle branch block with anterior STEMI. She was brought to the CCL emergently and  her initial systolic central blood pressure was 50 mm Hg. Left coronary angiography demonstrated occlusive thrombus extending across the length of the left main coronary artery with only TIMI 2 distal flow and minimal movement of the cardiac silhouette (Figure 3). As her hemodynamics continued to deteriorate, manual guide catheter aspiration and guidewire manipulation dislodged the thrombus with minimal thromboembolism to the apical LAD (Figure 4). Her cardiac motion on fluoroscopy
her initial systolic central blood pressure was 50 mm Hg. Left coronary angiography demonstrated occlusive thrombus extending across the length of the left main coronary artery with only TIMI 2 distal flow and minimal movement of the cardiac silhouette (Figure 3). As her hemodynamics continued to deteriorate, manual guide catheter aspiration and guidewire manipulation dislodged the thrombus with minimal thromboembolism to the apical LAD (Figure 4). Her cardiac motion on fluoroscopy 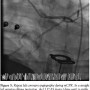 improved, but her blood pressure continued to decline and she required CPR for PEA cardiac arrest. Mechanical CPR was initiated and an IABP was placed via the right femoral artery. Intensive support with mCPR, IABP, and vasopressors provided full support for over 40 minutes as she experienced recurrent PEA and refractory ventricular fibrillation. Angiography during mCPR demonstrated patency of the left coronary artery (Figure 5). After continued support and intravenous lidocaine, her pulse was restored and the mCPR was discontinued. She was alert and responsive, and transferred to the coronary care unit (CCU) with IABP and vasopressor support. The IABP was removed on hospital day 2, although she continued to require inotropic support. Echocardiography demonstrated a normal-sized but severely hypokinetic left ventricle with an ejection fraction <20%. On hospital day 3, she experienced acute mental status changes due to an intracranial hemorrhage and expired on hospital day 4.
improved, but her blood pressure continued to decline and she required CPR for PEA cardiac arrest. Mechanical CPR was initiated and an IABP was placed via the right femoral artery. Intensive support with mCPR, IABP, and vasopressors provided full support for over 40 minutes as she experienced recurrent PEA and refractory ventricular fibrillation. Angiography during mCPR demonstrated patency of the left coronary artery (Figure 5). After continued support and intravenous lidocaine, her pulse was restored and the mCPR was discontinued. She was alert and responsive, and transferred to the coronary care unit (CCU) with IABP and vasopressor support. The IABP was removed on hospital day 2, although she continued to require inotropic support. Echocardiography demonstrated a normal-sized but severely hypokinetic left ventricle with an ejection fraction <20%. On hospital day 3, she experienced acute mental status changes due to an intracranial hemorrhage and expired on hospital day 4.
 Case 3. A 66-year-old woman with a history of end-stage renal disease with failed cadaveric renal transplant, diabetes mellitus, and hypertension collapsed at home and EMS was activated. She suffered recurrent PEA cardiac arrest in the field and after arrival to the emergency department at a local hospital. After restoration of circulation, her ECG demonstrated an acute inferior STEMI and she was transferred to our facility for primary PCI. The patient suffered recurrent PEA upon arrival in the CCL and mCPR was initiated. Left coronary angiography was performed during mCPR showing collateral filling of the RCA up to its ostial segment (Figure 6). Her right coronary artery was occluded at the origin and could not be engaged with a guiding catheter despite multiple attempts. Because of recurrent episodes of PEA, long cumulative CPR duration, and a poor prognosis, no further attempts at RCA PCI were made. After additional vasopressors and mCPR, she regained spontaneous circulation and was transferred to the CCU. The patient’s requirement for vasopressor support increased and she expired 3 hours later.
Case 3. A 66-year-old woman with a history of end-stage renal disease with failed cadaveric renal transplant, diabetes mellitus, and hypertension collapsed at home and EMS was activated. She suffered recurrent PEA cardiac arrest in the field and after arrival to the emergency department at a local hospital. After restoration of circulation, her ECG demonstrated an acute inferior STEMI and she was transferred to our facility for primary PCI. The patient suffered recurrent PEA upon arrival in the CCL and mCPR was initiated. Left coronary angiography was performed during mCPR showing collateral filling of the RCA up to its ostial segment (Figure 6). Her right coronary artery was occluded at the origin and could not be engaged with a guiding catheter despite multiple attempts. Because of recurrent episodes of PEA, long cumulative CPR duration, and a poor prognosis, no further attempts at RCA PCI were made. After additional vasopressors and mCPR, she regained spontaneous circulation and was transferred to the CCU. The patient’s requirement for vasopressor support increased and she expired 3 hours later.
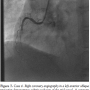 Case 4. A 75-year-old woman with a past history of hypertension and diabetes presented with 3 hours of unrelenting chest pain with associated hypotension. After pre-hospital ECG confirmed inferior STEMI, she was brought to our facility by EMS for primary PCI. While awaiting transfer to the CCL, she experienced her first PEA cardiac arrest with successful return of spontaneous circulation (ROSC) after CPR, epinephrine, and atropine. She experienced recurrent PEA arrest immediately prior to and during angiography and PCI. The patient received
Case 4. A 75-year-old woman with a past history of hypertension and diabetes presented with 3 hours of unrelenting chest pain with associated hypotension. After pre-hospital ECG confirmed inferior STEMI, she was brought to our facility by EMS for primary PCI. While awaiting transfer to the CCL, she experienced her first PEA cardiac arrest with successful return of spontaneous circulation (ROSC) after CPR, epinephrine, and atropine. She experienced recurrent PEA arrest immediately prior to and during angiography and PCI. The patient received 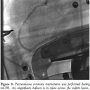 manual CPR and was intubated. Coronary angiography demonstrated a thrombotic culprit mid RCA occlusion (Figure 7). PTCA was performed during continuous mCPR (Figure 8) with subsequent ROSC. A bare-metal stent was placed in the mid RCA with restoration of TIMI 3 flow. An IABP was placed following left coronary angiography and the patient was transferred to the CCU on vasopressors. Despite increasing pressor support, the patient expired 12 hours later with a progressive sepsis-like syndrome.
manual CPR and was intubated. Coronary angiography demonstrated a thrombotic culprit mid RCA occlusion (Figure 7). PTCA was performed during continuous mCPR (Figure 8) with subsequent ROSC. A bare-metal stent was placed in the mid RCA with restoration of TIMI 3 flow. An IABP was placed following left coronary angiography and the patient was transferred to the CCU on vasopressors. Despite increasing pressor support, the patient expired 12 hours later with a progressive sepsis-like syndrome.
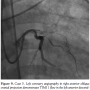 Case 5. A 57-year-old female with a history of diabetes mellitus, hypertension, and hyperlipidemia presented with a 3-month history of exertional angina. A diagnostic angiogram demonstrated a calcified 80% lesion in the mid left anterior descending (LAD) artery and 70% proximal circumflex lesion. The patient was referred to our institution for PCI. Her PCI was complicated by stent embolization and dissection of the proximal LAD and circumflex. The patient developed severe chest pain and angiography demonstrated TIMI 1 flow in the
Case 5. A 57-year-old female with a history of diabetes mellitus, hypertension, and hyperlipidemia presented with a 3-month history of exertional angina. A diagnostic angiogram demonstrated a calcified 80% lesion in the mid left anterior descending (LAD) artery and 70% proximal circumflex lesion. The patient was referred to our institution for PCI. Her PCI was complicated by stent embolization and dissection of the proximal LAD and circumflex. The patient developed severe chest pain and angiography demonstrated TIMI 1 flow in the  LAD (Figure 9). The patient suffered cardiac arrest with recurrent ventricular fibrillation requiring defibrillation 15 times. Mechanical CPR was initiated and continued during 30 minutes of persistent cardiac arrest while an IABP was placed and PCI of the LAD and circumflex were performed (Figure 10). TIMI 3 flow was achieved after placing 2 drug-eluting stents from the proximal to mid LAD and an additional stent in the circumflex. The patient regained spontaneous circulation with minimal pressor support and she was transferred to the CCU. Her condition improved and both the vasopressor and IABP were discontinued the following day. On hospital day 4, the patient was discharged home without any sequelae.
LAD (Figure 9). The patient suffered cardiac arrest with recurrent ventricular fibrillation requiring defibrillation 15 times. Mechanical CPR was initiated and continued during 30 minutes of persistent cardiac arrest while an IABP was placed and PCI of the LAD and circumflex were performed (Figure 10). TIMI 3 flow was achieved after placing 2 drug-eluting stents from the proximal to mid LAD and an additional stent in the circumflex. The patient regained spontaneous circulation with minimal pressor support and she was transferred to the CCU. Her condition improved and both the vasopressor and IABP were discontinued the following day. On hospital day 4, the patient was discharged home without any sequelae.
Discussion
Critically ill patients with either acute STEMI or resuscitated cardiac arrest will likely comprise a larger portion of catheterization laboratory patients in the future. Improved recognition of STEMI and timely primary PCI will feed this trend. Specialized treatment following cardiac arrest in dedicated centers will become more common. In this context, mCPR may prove itself to be an efficient, effective, and practical tool for resuscitation in the CCL. Mechanical CPR has been studied in various settings and with devices other than the LUCAS.
Placing an IABP or other ventricular assist device in a pulseless patient can be extremely cumbersome. Rescuers performing manual CPR in the CCL are directly in the field of view and exposed to radiation. This interferes with imaging, requiring frequent interruptions in CPR to accommodate various procedural maneuvers. Thus, effective and sustained manual CPR is difficult if not impossible to achieve. Mechanical CPR can be continued during placement of an IABP or other assist device, coronary angiography, and PCI. The current LUCAS device is easy to set up and operate with basic instruction.
Fluoroscopic and cineangiographic imaging during mCPR with the LUCAS device requires few significant adjustments. The images presented here represent one center’s experience and diagnostic images were obtained in all cases. Anterior-posterior projections are limited by the height and radio-opacity of the LUCAS drive unit over the patient’s chest. Straight right and left anterior oblique views provide adequate visualization despite the back-brace component of the device being visible on fluoroscopy. As evidenced in Cases 1 and 4, significant cranial angulation (>30°) can provide coronary imaging without visual encroachment by the device components.
In animal models, cerebral blood flow during CPR is improved with mCPR,15 and mCPR devices provide significantly higher coronary perfusion pressures than manual CPR.16 Coronary flow assessment has been reported in both animal models and human subjects.16,17 In 4 out of 6 patients studied by Larsen et al with TIMI 3 flow during mCPR, invasive measurements documented adequate coronary perfusion pressure gradients. In the present experience, coronary flow during mCPR is pulsatile, but classification by TIMI grade is readily apparent. In Cases 1, 4, and 5, the PCI procedure itself was performed during mCPR compressions (Figures 1, 2, 8, and 10).
The largest series detailing use of LUCAS mCPR during coronary angiography and PCI is from Wagner et al.14 This series does not focus on imaging or image quality, though it very clearly portrays the dire outcomes of patients suffering cardiac arrest as a complication of STEMI. In that series, 43 patients experiencing cardiac arrest in the CCL were treated with mCPR. PCI was attempted in 36 patients and was deemed successful in 27 of these patients. The average time of LUCAS support was over 28 minutes. Only 11 of the 43 critically ill patients survived to hospital discharge in good condition. Similarly, 4 out of 5 patients reported in our series did not survive to hospital discharge. Although the issues surrounding resource utilization in this population are serious, they are not the focus of this report. Nonetheless, caring for a large population with STEMI or following cardiac arrest makes these scenarios relatively common and high-quality CPR is of paramount importance as we strive to improve results.
Conclusion
Data regarding coronary angiography and PCI during mCPR have been provided by multiple European centers,10,14,18 but this is the first published report of use in North America. A few publications have included examples of coronary imaging in this setting.17,19 The use of the LUCAS device for mCPR provides the benefit of uninterrupted, sustained, high-quality chest compressions without requiring medical providers to work in the imaging field. This report demonstrates the ability to obtain clear and diagnostic angiographic images during mCPR. Interventional cardiologists will continue to struggle with the challenges of in-lab cardiac arrest, and mCPR may facilitate reaching the goal of improved patient outcomes.
References
- Farb A, Tang AL, Burke AP, Sessums L, Liang Y, Virmani R. Sudden coronary death. Frequency of active coronary lesions, inactive coronary lesions, and myocardial infarction. Circulation. 1995;92(7):1701-1709.
- Leach IH, Blundell JW, Rowley JM, Turner DR. Acute ischemic lesions in death due to ischemic heart disease. An autopsy study of 333 cases of out-of-hospital death. Eur Heart J. 1995;16(9):1181-1185.
- Virkkunen I, Paasio L, Ryynänen S, et al. Pulseless electrical activity and unsuccessful out-of-hospital resuscitation: what is the cause of death? Resuscitation. 2008;77(2):207-210.
- Dumas F, Cariou A, Manzo-Silberman S, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv. 2010;3(3):200-207.
- Garot P, Lefevre T, Eltchaninoff H, et al. Six-month outcome of emergency percutaneous coronary intervention in resuscitated patients after cardiac arrest complicating ST-elevation myocardial infarction. Circulation. 2007;115(11):1354-1362.
- Wolfrum S, Pierau C, Radke PW, Schunkert H, Kurowski V. Mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST-segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention. Crit Care Med. 2008;36(6):1780-1786.
- Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 Suppl 3):S640-S656.
- Maynard C, Rao SV, Gregg M, et al. The role of out-of-hospital cardiac arrest in predicting hospital mortality for percutaneous coronary interventions in the Clinical Outcomes Assessment Program. J Invas Cardiol. 2009;21(1):1-5.
- Ornato JP, Peberdy MA, Tadler SC, Strobos NC. Factors associated with occurrence of cardiac arrest during hospitalization for acute myocardial infarction in the second national registry of myocardial infarction in the U.S. Resuscitation. 2001;48(2):117-123.
- Wyss CA, Fox J, Franzeck F, et al. Mechanical versus manual chest compression during CPR in a cardiac catheterization setting. Cardiovasc Med. 2010;13(3):92-96.
- Hallstrom A, Rea TD, Sayre MR, et al. Manual chest compression vs use of an automated chest compression device during resuscitation following out-of-hospital cardiac arrest. JAMA. 2006;295(22):2620-2628.
- Ong MEH, Ornato JP, Edwards DP, et al. Use of an automated, load-distributing band chest compression device for out-of-hospital cardiac arrest resuscitation. JAMA. 2006;295(22):2629-2637.
- Lewis RJ, Niemann JT. Manual vs device-assisted CPR: reconciling apparently contradictory results. JAMA. 2006;295(22):2661-2664.
- Wagner H, Terkelsen CJ, Friberg H, et al. Cardiac arrest in the catheterization laboratory: a 5-year experience of using mechanical chest compressions to facilitate PCI during prolonged resuscitation efforts. Resuscitation. 2010;81(4):383-387.
- Rubertsson S, Karlsten R. Increased cortical cerebral blood flow with LUCAS: a new device for mechanical chest compressions compared to standard external compressions during experimental cardiopulmonary resuscitation. Resuscitation. 2005;65(3):357-363.
- Liao Q, Sjoberg T, Paskevicius A, Wohlfart B, Steen S. Manual versus mechanical cardiopulmonary resuscitation. An experimental study in pigs. BMC Cardiovasc Disord. 2010;10:53.
- Larsen AI, Hjornevik A, Bonarjee V, Barvik S, Melberg T, Nilsen DW. Coronary blood flow and perfusion pressure during coronary angiography in patients with ongoing mechanical chest compression: a report of 6 cases. Resuscitation. 2010;81(4):493-497.
- Grogaard HK, Wik L, Eriksen M, Brekke M, Sunde K. Continuous mechanical chest compressions during cardiac arrest to facilitate restoration of coronary circulation with percutaneous coronary intervention. J Am Coll Cardiol. 2007;50(11):1093-1094.
- Bonnemeier C, Olivecrona G, Simonis G, et al. Automated continuous chest compression for in-hospital cardiopulmonary resuscitation of patients with pulseless electrical activity: a report of five cases. Int J Cardiol. 2009;136(2):E39-E50.
__________________________________________
From the 1Division of Cardiology and 2Department of Emergency Medicine, Harbor UCLA Medical Center, Torrance, California.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted September 27, 2011, final version accepted October 4, 2011.
Address for correspondence: Joseph L. Thomas, MD, FSCAI, Assistant Clinical Professor of Medicine — David Geffen UCLA School of Medicine, Division of Cardiology, Harbor UCLA Medical Center, 1000 West Carson Street, RB-2, Torrance, CA 90509. Email: jthomas@labiomed.org
















