Novel Reconstructive Ladder for Reestablishing Functional Skin Graft Coverage in Chronic Lower Extremity Wounds
© 2023 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Abstract
Background. Chronic lower extremity (LE) wounds frequently require significant interventions to close. The success of any method depends on an adequately prepared wound bed, while factors including wound size, perfusion, contamination, or exposed tissue structures can thwart efforts. We propose a standardized algorithm of care utilizing an acellular dermal matrix, split-thickness skin graft (STSG), and negative pressure wound therapy (NPWT) for the treatment of LE wounds.
Methods. This was a single-center, retrospective cohort study examining patients who underwent LE wound debridement, placement of fetal bovine dermis (FBD), and STSG between 2016 and 2022. The primary outcome was wound closure, while secondary outcomes were wound infection and amputation-free survival.
Results. Twenty patients (mean age 59 years, M:F 12:8)—including 24 LE venous ulcers (29.4%), amputation sites (29.4%), diabetic foot ulcers (25.0%), and atypical wounds (16.7%) with an average area of 39.15 cm2—underwent debridement and FBD placement followed by STSG a median of 61 days thereafter. Of these patients, 83.3% received NPWT after FBD and STSG with 86% closure. There was successful engraftment in 92% of wounds whose FBD placement was within 2 months of STSG. Of wounds that had <50% engraftment, 75% had a STSG placed over 2 months after FBD placement. Of those patients with post-STSG infection, 75% had the graft placed >2 months after FBD placement, one of which required proximal amputation.
Conclusions. By following a treatment plan including debridement with treatment of infection, application of FBD with placement of STSG within 2 months thereafter, and reinforcing NPWT, chronic wounds will have an increased rate of successful reepithelialization. Many cases experienced delays from FBD engraftment until STSG application due to schedule and insurance impediments, which led to less favorable outcomes. Therefore, a protocol that involves scheduling the placement of STSG 4 weeks after successful engraftment of FBD has been adopted.
Introduction
The goal of chronic wound management is to facilitate healing, which often encompasses many strategies of various iterations, such as the use of diverse dressings, antimicrobials, and/or operative interventions.1 These treatment options can differ depending on several factors, such as the size of the wound, tissue structures exposed, level of contamination, and degree of vascular perfusion. While a variety of these treatment strategies have been deemed effective, the clinical indications, modes of action, and use alone or conjointly are not clearly outlined.2
Rotational flaps, free flaps, full-thickness skin grafts, or split-thickness skin grafts (STSG) are just a few of the many methods used to close chronic wounds.3 Moreover, cellular or noncellular biologic products, fabricated collagen foams, hyperbaric oxygen therapy, and negative pressure wound therapy (NPWT) can additionally be used to enhance various treatments.2
Traditionally, STSGs were the gold standard of wound healing, both to primarily close a well-granulated chronic lower extremity wound, or as an adjunctive therapy for wounds that partially closed in response to other therapies. The amount of donor availability, ability to be meshed to cover large surface areas, and the ability of the donor site to heal spontaneously by epithelization make STSGs desirable.3 Despite their widespread use, however, STSGs frequently fail when used alone as they are highly dependent on the quality of the wound bed to which they are placed. It has been proven that poor vascularity, a large surface area, high bacterial bioburden, and/or excessive shearing forces can thwart successful engraftment.4 Additionally, STSG alone as a treatment does not account for the adequate redevelopment of a functional dermis.
The field of tissue engineering and NPWT have emerged as auspicious solutions to minimize the problems associated with skin transplantation, such as scar formation, poor cosmesis, and rejection. In fact, many cellular and tissue-based bioengineered skin substitutes are currently available, including a plethora of porcine or bovine xenografts and dermal substitutes. The properties of these products vary, such as composition, cost, and technique of application, although all serve as a barrier against microorganisms and as a means to promote healthy granulation tissue and accelerate wound healing.5 Here, we focus on a specific fetal bovine dermis, Primatrix, as it has been well tolerated and proven effective among wounds of various etiologies.
Augmentation with negative pressure wound therapy (NPWT) or conventional bolster dressings are often used as an adjunct to both cellular-based tissue products or STSG placement to prepare the wound beds for grafting or flap closure by securing the tissue/product in place, removing edema, and increasing perfusion. NPWT has been reported as a good alternative to conventional dressings for STSGs, as it has been shown to increase the rate of graft take and decrease the reoperation rate.6
Despite the achievements and advancements in the biochemical development of wound care, there is no clear standard of care defining what method(s) to use and when to optimize results. Thus, we propose a 3-part reconstructive ladder of care, utilizing both an acellular fetal bovine dermal matrix (FBD) and STSG for the treatment of lower extremity (LE) diabetic ulcers, venous stasis wounds and atypical wounds secondary to lupus, rheumatoid arthritis, pyoderma gangrenosum, protein C deficiency, sickle cell anemia, Charcot foot, and exposed Achilles tendons. Specifically, we believe that by priming a well-excised wound with fetal bovine collagen and NPWT, followed by placement of an autologous STSG within 2 months (ideally 4 weeks) of FBD application with 4 days of additional NPWT, these chronic LE wounds will have an increased rate of successful re-epithelialization.
Materials and Methods
This was a single-center, retrospective cohort study examining patients who underwent a lower extremity wound debridement, placement of a FBD (Primatrix), and interval STSG between February 2016 to January 2022. The inclusion criteria were all patients with full-thickness wounds (requiring removal of necrotic dermis), independent of etiology. Data were collected using Current Procedural Terminology codes to identify those who underwent placement of skin substitutes and STSGs. Only those who underwent prior Primatrix placement to the same location as the STSG were included in our cohort, while patients who solely received a FBD or STSG to a single wound were excluded from our study. Additionally, the patients needed to be compliant with treatment of their underlying pathophysiology: patients with diabetes were required to comply with offloading, and patients with venous leg ulcer patients were required to adhere to compression therapy. Duration between revascularization and history of venous intervention were not factors in the inclusion or exclusion criteria.
All patients who presented with clinical signs of a wound infection received X-rays to evaluate osteomyelitis (OM), an associated abscess or cellulitis. If there was any ambiguity about the presence of infection, magnetic resonance imaging or computed tomography was performed, and margins were taken via sharp resection (bone saw) during debridement. In this cohort of patients, those with wounds under 40 cm2 were debrided with ultrasound-enhanced curettage, while those greater than 40 cm2 were debrided with tangential hydro-surgery. Patients were treated with antibiotics tailored to their culture taken at the bone margin(s): 14 days if the culture showed no growth and 6 weeks if the margin was positive. All wounds were actively free of infection or being actively treated for infection at the time of debridement and FBD placement.
All patients received the same FBD: Primatrix (Integra LifeSciences) acellular collagen matrix. We chose to use Primatrix because it is a well-studied, porous, nutrient-rich xenograft that enhances cell infiltration, rapid engraftment, and creation of a neodermis that can support a STSG.7,8 Additionally, it has been well tolerated and proven effective to its counterparts, requiring only a single application with resistance to bacterial degeneration, unlike almost all other xenografts.9 Likewise, it is unique in its composition of type I and III collagen found only in fetal tissue and has been shown to successfully assimilate into a variety of wound types.10 Typically, it engrafts (is accepted by the host, with endogenous tissue moving through the graft) over the immediate 30 days.


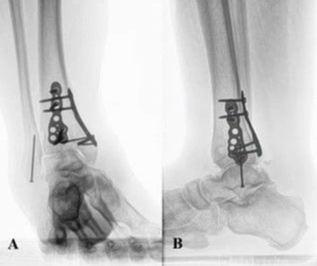
After FBD placement (Figure 1), either a bolster dressing or NPWT was placed. For all patients who received NPWT (3M Corp), we used the same traditional inpatient model of continuous negative pressure of 125 mm Hg for 4 days after FBD placement, after which a moisture retentive dressing was applied (Figure 2). The patients were then either discharged to a subacute rehabilitation (SAR) facility or home with perioperative visiting nurse services. Outpatient follow-up appointments were made for 1 to 2 weeks after surgery at the time of discharge to evaluate preparedness for interval STSG, although these appointments were occasionally missed or rescheduled.




Parameters used to ascertain readiness for STSG included healthy granulation tissue devoid of scar tissue, eschar, or infection (Figures 3 and 4). No histologic nor biopsy data was obtained, and the decision to proceed to skin graft was made based on clinical grounds alone. Once seen in the office and interval STSG was booked and performed (Figure 5), patients were again discharged to an SAR facility or home after 4 days of the same NPWT (Figure 6) with visiting nurse services. Postoperative appointments were scheduled for 1 to 2 weeks after STSG. The general follow-up schedule in the office was every week thereafter for both the application of FBD and STSG, although outcome variables were assessed at the first postoperative visit despite the variation in actual days since the intervention.
The primary outcome measure of our study was wound closure while secondary outcomes were wound infection and amputation-free survival after our treatment plan. Unique patient variables and comorbidities were additionally evaluated as possible confounding variables. All variables were categorical and described using both percentages and proportions. No statistical software was used for analysis.
Results
Twenty patients including 24 LE wounds underwent debridement and placement of FBD followed by STSG. The average age of the cohort was 59 years, and the male to female ratio was 12:8. The distribution of wounds were venous ulcers (29.4%), amputation sites (29.4%), diabetic foot ulcers (25.0%), and atypical wounds, including Charcot foot ulcers and exposed Achilles tendons (16.7%). The average area of involvement was 39.15 cm2 (range, 3.2-110.0 cm2). The median and mean time to first follow-up was 11 days and 19 days after STSG, respectively.
In the cohort, 80% had poorly controlled diabetes with a mean hemoglobin A1c of 9.6%. There was no significant correlation between hemoglobin A1c and percent engraftment, though many diabetic patients remained on perioperative antibiotics for least 14 days, depending upon the results of cultures from their surgical margins. Approximately 55% of patients initially presented with acute OM or a soft tissue infection. Of those, there was an even distribution between bone and soft tissue, and 100% were treated with surgical debridement and antibiotics with bone margins taken and analyzed for the histologic and culture evidence of OM. FBD was most often placed at the time of initial debridement. Before FBD placement, 40% had known peripheral artery disease with documented ankle-brachial index at the time of initial presentation; all patients had an ankle-brachial index >0.7 before placement of FBD.
After initial debridement and FBD placement (Figure 1), 50% of patients were discharged to a SAR facility, and the remaining 50% were discharged to home with visiting nurse services. Subsequent STSGs were performed at a median of 61 days (mean, 64 days; range, 21-188 days) after FBD placement once deemed ready during an office visit assessment; parameters included healthy granulation tissue devoid of scar, eschar, or infection (Figures 3 and 4). Most (83.3%) patients received 4 days of NPWT after both FBD and STSG placement (Figures 2 and 6), while the remaining 15.6% received a bolster dressing. Patients who received bolster dressings had an average of 80% closure at the first postoperative visit, whereas patients who received NPWT had an average of 86% closure.
Approximately 92% of wounds that received a STSG within 2 months of FBD placement had successful adherence, defined as successful inosculation and revascularization by first postoperative appointment. One wound (4.2%) failed to meet this parameter and reopened without any engraftment during this immediate time frame because of primary chronic vasculitis. Approximately 75% wounds that had <50% take on evaluation at the first office visit after STSG were in patients who underwent STSG placement over 2 months after FBD placement. There was no significant correlation between history of diabetes, wound size, wound type, or noninfected state and those wounds with 100% graft take at first post-STSG visit. There were no documented instances of hypertrophic scars or keloids by each first postoperative appointment nor any instances of acute infections or emergent amputations. There was 1 report of newly diagnosed chronic OM at first postoperative appointment secondary to persistent exposed bone that was treated with additional antibiotics. Insurance difficulties and follow-up scheduling issues were the reasons for patients whose STSG was delayed more than 4 weeks after successful FBD placement.
While our study mostly focuses on graft take in the immediate postoperative period, the long-term follow-up has been documented out to 6.3 years (with a mean and median of 2.7 and 2.3 years, respectively). Two patients were lost to follow-up after their first post-STSG outpatient appointment, and no long-term data are available for 5 patients after close follow-up during the first postoperative year. Of the 20 patients who were included in our study, 4 died; all 4 deaths were unrelated to their LE wounds (ischemic cardiomyopathy [n = 1], diabetic ketoacidosis [n = 1], and respiratory failure [n = 2]). In the patients with deep diabetic foot wounds, 4 wounds (16.7%) developed recurrent acute OM or an abscess requiring antibiotics within 2 years after STSG. Approximately 75% of those patients with post-STSG infection had their graft placed >2 months after FBD placement.


Three patients (12.5%) ultimately had amputations proximal to STSG for critical limb ischemia or chronic OM, all occurring approximately 3.5 years after STSG. Of those patients requiring amputation, the 2 patients with critical limb ischemia had their STSG placed <2 months after FBD placement whereas the third patient with chronic OM received their STSG >2 months after FBD placement. Of those who did not require a proximal amputation or were not lost to follow-up, 2 patients experienced wound reopening because of arterial/venous insufficiency, and 1 patient had their wound reopen because of noncompliance with offloading. Approximately 73% of wounds assessed at more than 1 postoperative appointment healed well with long-term viability and excellent functionality. The transmetatarsal wounds treated with this therapy are the only instances of some long-term hypertrophic scarring (Figure 7). In general, return to weight-bearing status and use of appropriate footwear has been excellent. Please see Figures 8 through 13 for additional images describing the normal treatment course and outcome.







Discussion
Wounds that do not heal in an orderly fashion are unfortunately common and often incorrectly treated.11 The high morbidity, financial burden, and negative psychological effects associated with these wounds further highlight the importance of implementing routine guidelines. Obviously, chronic wounds need treatment algorithms that take into consideration the variations in problems encountered. Currently, there is no standard approach to the treatment of nonhealing lower extremity wounds as overcoming the pathophysiology of delayed healing in complex patients is challenging. As a result, physicians and health care practitioners are often faced with considering a myriad of possible treatment options, each with its own pros and cons.
Generally, most wounds are treated with some form of tissue debridement, infection control, moisture balance, and edge approximation. Treatment approaches tend to deviate further, depending on the ulcer type. For example, diabetic ulcers are managed with offloading while venous ulcers are treated with compression and leg elevation.11 Sometimes, deeper wounds that expose vital structures require adjacent soft tissue coverage or potentially even a free flap; however, none of the patients in the retrospective review had such structures other than bone and tendon exposed. In our study, we found a way to treat nonhealing wounds of various etiologies successfully and uniformly. This approach was used in conjunction with appropriate primary therapy, such as offloading and compression.
Debridement has always been an integral component of wound healing as it removes nonviable tissue and biofilms that prolong the inflammatory response and impede quick and successful wound healing. In short, it turns a chronic wound into an acute wound, which kick-starts a fresh healing process. While sharp debridement is traditionally the standard of care, hydrosurgical or ultrasonic devices have recently proven effective as well, both in the operating room and outpatient clinic.12,13 Regardless of the method used, proper initial debridement remains a paramount component of the wound healing process, and the reason why we emphasize it in our algorithm.
Extracellular matrices (ECMs), such as FBD, have gained popularity in recent years as they promote neovascularization and the development of a neodermis that serves as a nutrient-rich scaffold for STSGs within 2 to 4 weeks of placement.14 While most ECMs are often biocompatible, user friendly, and safe, the differences in source species, tissues, and manufacturing processes can alter the wound healing process, emphasizing the importance of product choice, such as FBD, in surgical preparation.
Since its introduction in the United States in 1997, NPWT has become an integral component of wound healing by accelerating the incorporation of an ECM on a wound bed. NPWT provides a controlled, continuous or intermittent subatmospheric pressure that increases the rate and success of revascularization.15 It has been recognized as a method to reduce dressing changes, decrease the time between serial debridements, and reduce costs of a prolonged hospital admissions.16,17 Multiple randomized clinical trials, such as those led by Armstrong and Lavery et al, have further demonstrated the efficacy of NPWT as opposed to advanced moist wound therapy, with NPWT use achieving better granulation in a shorter time frame.18 NPWT after FBD placement and STSG is therefore of utmost importance as it enhances engraftment and acts as a very functional bolster.
When using dermal templates, wounds greater than 4 cm2 often require a STSG to enhance closure. After successful engraftment of a dermal template, autologous skin is applied to the wound bed to aid in re-epithelization. These wound beds must be well granulated and healthy because STSGs do not have their own blood supply and must rely on a well-vascularized wound bed to work. Once placed, these grafts require 96 hours to complete proper imbibition and inosculation, often aided by bolster dressings or NPWT; however, full maturation can take up to 1 year.19 The goal of this process is to provide a near-functional and robust wound closure that will stand up to long-term repetitive trauma.
By combining all the aforementioned aspects of optimal wound healing, we propose a standardized protocol for successful chronic wound closure. While medical treatment that includes such goals as improved glucose control and smoking cessation can certainly improve outcomes, we discovered that applying a standardized treatment plan proves successful regardless of patient comorbidities. However, we continue to aggressively support long-term follow-up with compression and offloading as appropriate. This standardization of care requires strict inclusion and exclusion criteria; patients must be compliant with postoperative instructions, including wound care and offloading. Close outpatient follow-up and timely STSG placement is also needed for optimal results.
Limitations
Our study has several limitations, including the small sample size and single-center retrospective design. Our ability to evaluate and characterize long-term success is limited by the retrospective nature of this study and time frame during which results were analyzed. Additionally, the data used in this study were derived from a database of all patients who received FBD and adhered to our protocol, thus representing a selection bias without a proper control group. Moreover, several patients were lost to follow-up after their intervention or had a delay between procedures because of scheduling conflicts or insurance issues, which were deviations from our proposed protocol.
Conclusions
In conclusion, while STSGs can take up to 1 year for full maturation, we found successful short-term results, including a large engraftment, by the first postoperative visit after we adopted a standardized 3-step treatment plan:1) initial debridement with treatment of infection; 2) application of FBD followed by 4 days of NPWT; and 3) placement of STSG within 2 months of FBD application, followed by an additional 4 days of NPWT. Under this protocol, chronic wounds demonstrated an increased rate of successful re-epithelialization, even in those patients with poor glucose control or initial active infection. However, basic tenets of care still need to be enforced and routinely followed for more favorable results. Cases delayed from FBD engraftment until STSG application due to schedule and insurance impediments often had less favorable outcomes. Therefore, a protocol that involves scheduling the placement of STSG 4 weeks after successful engraftment of FBD has been adopted by the authors.
References
1. O’Meara S, Cullum N, Majid M, Sheldon T. Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration. Health Technol Assess. 2000;4(21):1-237.
2. Hayn E. Successful treatment of complex traumatic and surgical wounds with a foetal bovine dermal matrix. Int Wound J. 2014;11(6):675-680. doi:10.1111/iwj.12028
3. Simman R. Wound closure and the reconstructive ladder in plastic surgery. J Am Col Certif Wound Spec. 2009;1(1):6-11. doi:10.1016/j.jcws.2008.10.003
4. Snyder RJ, Fife C, Moore Z. Components and quality measures of DIME (devitalized tissue, infection/inflammation, moisture balance, and edge preparation) in wound care. Adv Skin Wound Care. 2016;29(5):205-21 doi:10.1097/01.ASW.0000482354.01988.b4 5.
5. Oualla-Bachiri W, Fernández-González A, Quiñones-Vico MI, Arias-Santiago S. From grafts to human bioengineered vascularized skin substitutes. Int J Mol Sci. 2020;21(21):8197. doi:10.3390/ijms21218197
6. Yin Y, Zhang R, Li S, Guo J, Hou Z, Zhang Y. Negative-pressure therapy versus conventional therapy on split-thickness skin graft: a systematic review and meta-analysis. Int J Surg. 2018;50:43-48. doi:10.1016/j.ijsu.2017.12.020
7. Parcells AL, Karcich J, Granick MS, Marano MA. The use of fetal bovine dermal scaffold (PriMatrix) in the management of full-thickness hand burns. Eplasty. 2014;14:e36.
8. Rennert RC, Sorkin M, Garg RK, Januszyk M, Gurtner GC. Cellular response to a novel fetal acellular collagen matrix: implications for tissue regeneration. Int J Biomater, 2013;2013:527957. doi:10.1155/2013/527957
9. Lantis JC, Snyder R, Reyzelman AM, et al. Fetal bovine acellular dermal matrix for the closure of diabetic foot ulcers: a prospective randomised controlled trial. J Wound Care. 2021;30(Sup7):S18-S27. doi:10.12968/jowc.2021.30.Sup7.S18
10. Lineaweaver W, Bush K, James K. Suppression of alpha smooth muscle actin accumulation by bovine fetal dermal collagen matrix in full thickness skin wounds. Ann Plast Surg. 2015;74(Suppl 4):S255-S258. doi:10.1097/SAP.0000000000000449
11. Bowers S, Franco E. Chronic wounds: evaluation and management. Am Fam Physician. 2020;101(3):159-166.
12. Granick MS, Tran BNN, Alvarez OM. Latest advances in wound debridement techniques. Surg Technol Int. 2020;36:37-40.
13. James CV, Patel M, Ilonzo N, et al. Hydrosurgical debridement use associated with decreased surgical site-related readmissions: a retrospective analysis. Wounds. 2021;33(6):139-142. doi:10.25270/wnds/032821.01
14. Hsu KF, Yu-Lung Chiu N, Chiao HY, et al. Negative-pressure wound therapy combined with artificial dermis (Terudermis) followed by split-thickness skin graft might be an effective treatment option for wounds exposing tendon and bone: a retrospective observation study. Medicine (Baltimore). 2021;100(14):e25395. doi:10.1097/MD.0000000000025395
15. Kunze KN, Hamid KS, Lee S, Halvorson JJ, Earhart JS, Bohl DD. Negative-pressure wound therapy in foot and ankle surgery. Foot Ankle Int. 2020;41(3):364-372. doi:10.1177/1071100719892962
16. Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. 2014;51(7):301-331. doi:10.1067/j.cpsurg.2014.04.001
17. Younan G, Ogawa, R, Ramirez M, Helm D, Dastouri P, Orgill DP. Analysis of nerve and neuropeptide patterns in vacuum-assisted closure-treated diabetic murine wounds. Plast Reconstr Surg. 2010;126(1):87-96. doi:10.1097/PRS.0b013e3181da86d0
18. Armstrong DG, Lavery LA, Boulton AJ. Negative pressure wound therapy via vacuum-assisted closure following partial foot amputation: what is the role of wound chronicity? Int Wound J. 2007;4(1):79-86. doi:10.1111/j.1742-481X.2006.00270.x
19. Braza ME, Fahrenkopf MP. Split-thickness skin grafts. StatPearls. StatPearls Publishing; 2023.