Auricular Cartilage Composite Graft for Glans Reconstruction After Squamous Cell Carcinoma of the Penis
Abstract
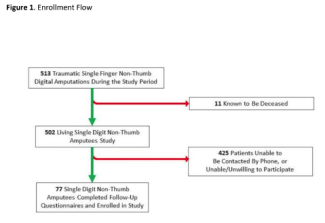
Background. Surgical procedures for squamous cell carcinoma of the penis generally involve primary closure, partial glansectomy, skin graft, and penile amputation. Partial penile resection can result in not only unsightly deformation of the penis but also functional disorders of the urinary line as well as psychological effects due to subjective perceptions of a loss of power and masculinity. With the use of an organ-preserving procedure for functional reconstruction without compromising oncological control, this report describes a new procedure for performing functional penile reconstruction with an auricular cartilage composite graft.
Introduction
Partial penile resection can result in not only unsightly deformation of the penis but also functional disorders of the urinary line as well as psychological effects due to subjective perceptions of a loss of power and masculinity. Auricular composite grafts are useful for glans reconstruction following surgical resection of penile cancer.
Methods and Results

A 92-year-old man was biopsied by a previous doctor for a penile glans mass that had developed 6 months prior and had been diagnosed as squamous cell carcinoma (SCC). The patient was referred for extended resection (Figure 1).

A relatively well-defined flat 12-mm tumor was located on the dorsal surface of the glans penis (T1b N0 M0 Stage II). An extended resection 10 mm away from the tumor margin was performed in the glans and penile corpus cavernosum, and the resection surface was covered with artificial dermis (Figure 2). The histopathological image showed that atypical squamous cells had infiltrated and were proliferating while adopting a supraclavicular structure accompanied by cancer pearl formation, and some intravascular tumor embolisms in the superficial corpus cavernosum were observed. The excision margin was negative (Figure 3).

No obvious distant metastasis was found by whole-body computed tomography, and absolute excision was confirmed by a pathological examination. A 10 mm-wide wedge-shaped full-thickness auricle was grafted onto the glans defect, and the penile shaft skin defect was covered by a full-thickness skin grafting from the inguinal region 5 weeks after extended resection had been performed (Figures 4 and 5).


The patient showed no urethral stricture 3 months after the operation and had achieved an appropriate glans morphology with high patient satisfaction. The texture of the transplanted auricular cartilage was not absorbed and was maintained, indicating the hardness of the glans crown at the time of erection. In addition, unlike simple skin graft surgery, the unique morphology was able to be reconstructed (Figure 6).

Discussion
Surgical procedures for SCC of the penis generally involve primary closure, partial glansectomy, skin graft, and penile amputation. Partial penile resection can result in not only unsightly deformation of the penis but also functional disorders of the urinary line and psychological effects due to subjective perceptions of a loss of power and masculinity.1
The local recurrence rate in the treatment of penile SCC is 4% (LE 2b) with partial/total penectomy, 14 to 20% (LE 3) with laser treatment, and 9.2% (LE 2b) with penile-preserving surgery. No significant difference in the rates of lymph node metastasis or cancer-specific survival have been reported between the groups with and without local recurrence.2
In recent years, organ-preserving procedures for functional reconstruction have been increasing without impairing oncological control. T1 lesions limited to the foreskin are recommended for wid4 local excision with circumcision; T1 lesions of the glans are recommended for primary closure, partial glansectomy, or a skin graft; and T2 lesions involving the glans are recommended for glansectomy with a skin graft and/or a local foreskin flap. In recent years, the surgical resection margin has been set at only a few millimeters, and there is a tendency to shift from highly invasive surgery such as penectomy to minimally invasive surgery.1,3,4 However, there are some penile cancers that require extensive extended resection, and some cases have undergone reconstruction with local flaps using penile skin or more complicated free flaps.5,6 Surgical procedures, including cartilage composite grafts, are commonly applied from the auricle to nasal wing defects, and they have proven to be very stable with high engraftment rates.7 The elasticity and morphology of the auricular cartilage shows morphological similarity to the corona of the glans. In addition, because the corpus cavernosum of the penis has an abundant blood flow, the engraftment rate of the auricular composite graft can be expected to be greater than that seen in nose reconstruction. The simplicity of this procedure and morphological similarity between the glans and auricle will result in a functionally useful penis with minimally invasive treatment. Because this case involved a patient of extremely old age, it did not lead to an erection; however, this procedure should be applied in the treatment of younger patients in the future.
Conclusions
Auricular composite grafts are useful for glans reconstruction following surgical resection of penile cancer.
Acknowledgments
Affiliations: Department of Plastic and Reconstructive Surgery, Kansai Medical University, Osaka, Japan
Correspondence: Masakatsu Hihara; hiharams@hirakata.kmu.ac.jp
Ethics: This study conforms to the Declaration of Helsinki ethical principles for medical research.
Disclosures: The authors have no relevant financial or nonfinancial interests to disclose.
References
1. Hakenberg OW, Compérat EM, Minhas S, Necchi A, Protzel C, Watkin N. EAU guidelines on penile cancer: 2014 update. Eur Urol. 2015;67(1):142-150. doi:10.1016/j.eururo.2014.10.017
2. Solsona E, Bahl A, Brandes SB, et al. New developments in the treatment of localized penile cancer. Urology. 2010;76(2 Suppl 1):S36-S42. doi:10.1016/j.urology.2010.04.009
3. Hegarty PK, Shabbir M, Hughes B, et al. Penile preserving surgery and surgical strategies to maximize penile form and function in penile cancer: recommendations from the United Kingdom experience. World J Urol. 2009;27(2):179-187. doi:10.1007/s00345-008-0312-x
4. Yang J, Chen J, Wu XF, et al. Glans preservation contributes to postoperative restoration of male sexual function: a multicenter clinical study of glans preserving surgery. J Urol. 2014;192(5):1410-1417. doi:10.1016/j.juro.2014.04.083
5. Yang J, Chen J, Wu XF, et al. Glans-reconstruction with preputial flap is superior to primary closure for post-surgical restoration of male sexual function in glans-preserving surgery. Andrology. 2014;2(5):729-733. doi:10.1111/j.2047-2927.2014.00239.x
6. Ahn DK, Kim SW, Park SY, Kim YH. Reconstructive strategy and classification of penoscrotal defects. Urology. 2014;84(5):1217-1222. doi:10.1016/j.urology.2014.06.023
7. Han DH, Mangoba DC, Lee DY, Jin HR. Reconstruction of nasal alar defects in asian patients. Arch Facial Plast Surg. 2012;14(5):312-317. doi:10.1001/archfacial.2012.520