Vitamin D: It Does a Body Good
Vitamin D has been dubbed by many as the “wonder drug of the 21st century.” While low vitamin D levels have long been correlated with diseases such as rickets and osteomalacia, research is increasingly linking vitamin D deficiency to more chronic illnesses, such as cancer, diabetes, and multiple sclerosis. Low vitamin D levels have also been linked to an increased risk of falls and fractures, which can be especially worrisome for nursing home residents. This article outlines vitamin D sources and physiology, research on pathology linked to vitamin D deficiency, assessment of vitamin D levels, treatment in those found to be deficient, and potential toxicities when levels are too high. (Annals of Long-Term Care: Clinical Care and Aging. 2010;18[11]:39-45.)
____________________________________________________________________________________________________________
Over the past two decades, there has been a significant increase in knowledge concerning many aspects of vitamin D, including the interrelationship between low vitamin D levels and many disease processes. Historically, rickets and osteomalacia have been the diseases most commonly associated with low vitamin D levels.1 Recently, more attention has been paid to other syndromes and disease processes associated with low vitamin D levels, such as multiple sclerosis, cancer, type 1 and type 2 diabetes, and increased risk of falls and fractures. In addition, overall mortality and cardiovascular mortality have been found to be inversely related to vitamin D levels.2 It appears that many seemingly disparate conditions are linked to vitamin D deficiency because most cells in the body have vitamin D receptors.3
Sources and Physiology
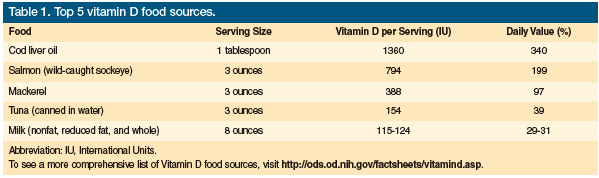
Vitamin D is a prohormone that is obtained either by ingestion or by skin exposure to ultraviolet B (UVB) light with a wavelength of 290 to 315 nm.4 It does not occur naturally in many foods ingested by most people, thus humans are largely dependent on foods fortified with vitamin D or on the manufacture of vitamin D by the skin after sun exposure. Vitamin D2, also called ergocalciferol, is present in certain fungi (eg, shiitake mushrooms), egg yolks, and some fatty fish, and is the form present in the supplements available by prescription. Vitamin D3, or cholecalciferol, is found in some fatty fish and in the over-the-counter supplements. Most of the vitamin D3 obtained from diet is from fortified foods, such as dairy products, margarine, and cereals.5 It is also the form manufactured by the skin after sun exposure. Table 1 outlines the top five vitamin D food sources.
Vitamin D3 is formed when UVB rays penetrate the skin, transforming 7-dehydrocholesterol to previtamin D3, which is then converted to vitamin D3 (Figure). There is a mechanism whereby any excess vitamin D3 is destroyed by sunlight so that vitamin D toxicity cannot occur from sun exposure.4 Both vitamin D2 from diet and vitamin D3 from diet and sun exposure are then metabolized in the liver to form 25-hydroxyvitamin D (25(OH)D). In the kidneys, 25(OH)D is metabolized to 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), or calcitriol, which is the active form of the vitamin. The rate of conversion to the active form is regulated by parathyroid hormone, calcium, and phosphorus levels.
 Historically, vitamin D was thought to have most of its effects on calcium and bone metabolism because it increases the active intestinal absorption of calcium and phosphorus, causes skeletal mineralization, regulates parathyroid hormone levels, and stimulates renal tubular calcium reabsorption. When calcium levels are lower due to insufficient vitamin D levels, parathyroid hormone levels increase, which stimulates the production of osteoclasts, causing increased bone resorption and an increased risk of fracture. However, because of the ubiquitous presence of vitamin D receptors in cells and tissue, many other disease processes and syndromes may be associated with vitamin D deficiency.3,6
Historically, vitamin D was thought to have most of its effects on calcium and bone metabolism because it increases the active intestinal absorption of calcium and phosphorus, causes skeletal mineralization, regulates parathyroid hormone levels, and stimulates renal tubular calcium reabsorption. When calcium levels are lower due to insufficient vitamin D levels, parathyroid hormone levels increase, which stimulates the production of osteoclasts, causing increased bone resorption and an increased risk of fracture. However, because of the ubiquitous presence of vitamin D receptors in cells and tissue, many other disease processes and syndromes may be associated with vitamin D deficiency.3,6
Vitamin D and Pathology
Calcitriol helps regulate more than 200 genes, accounting for vitamin D’s wide-ranging effects.7 Vitamin D receptors are located in most of the body’s cells, including musculoskeletal cells, vascular smooth muscle cells, endothelial cells, cardiomyocytes, activated T and B lymphocytes, neurons, oligodendrocytes, and astrocytes. In addition to being directly involved in bone health, calcitriol acts as a hormone that affects bile acid transport, renin production, and the endocrine system.3 Calcitriol also increases insulin production and myocardial contractility.1 Vitamin D helps modulate the immune system,4 impacts muscle function,8 and has antioxidative and anti-inflammatory effects.6,9
Vitamin D may impact inflammation through its modulation of cytokine production. A deficiency in vitamin D has been linked to increases in various inflammatory markers, including C-reactive protein, interleukin-10, interleukin-2, interferon-gamma, and tumor necrosis factor-alpha,10,11 whereas increased levels of vitamin D are positively associated with interleukin-4 and transporting growth factor beta.10 Calcitriol suppresses the release of cytokines by lymphocytes.11 A deficiency of calcitriol is positively associated with elevated levels of C-reactive protein and interleukin-6.11
Vitamin D’s role in influencing the immune system is through its effects on the growth and differentiation of cells, including macrophages, dendritic cells, and T and B lymphocytes.6,12 It also activates genes responsible for the regulation of cellular proliferation, apoptosis, and angiogenesis.1,12 Calcitriol inhibits invasiveness and metastatic potential.3 These effects could reduce the number of atheromatous lesions and reduce the growth of cancer cells.3 It is possible that extracellular concentration of 25(OH)D is an essential determinant of the ability of cells to regulate proliferation.4 Vitamin D immune influence extends into infectious processes. Calcitriol is also involved in the synthesis of cathelicidin, an antimicrobial polypeptide that is part of the innate immune response. It is capable of destroying Mycobacterium tuberculosis and other pathogens; however, when the level of 25(OH)D falls below 20 ng/mL, macrophages are unable to perform this function.13
Diabetes
Vitamin D impacts insulin production and, potentially, the production of islet autoantibodies; thus, vitamin D deficiency may play a role in the development of type 1 and type 2 diabetes.14 Polymorphisms in vitamin D receptors have been associated with type 1 diabetes.6,15 A longitudinal study of children taking vitamin D supplements showed an 80% reduction in the risk of developing type 1 diabetes.16
Vitamin D has been linked to type 2 diabetes because vitamin D deficiency has been found to lead to processes associated with an increased risk of this disease, including insulin insensitivity and cytokine regulation.6,17 Vitamin D impacts available calcium, which plays a role in insulin response to glucose load. It has been hypothesized that insulin resistance is triggered by a change in calcium that is available in insulin target tissues, which then interferes with insulin signal transduction.18 In the Nurses Health Study, which included over 83,000 women, those with daily calcium and vitamin D intake of more than 1200 mg and 800 IU, respectively, had a 33% lower risk of developing type 2 diabetes than those whose intake was less than 600 mg and 400 IU, respectively.19 A study based on data from the Third National Health and Nutrition Examination Survey (NHANES III) indicated an inverse relationship between 25(OH)D levels and type 2 diabetes (possibly resulting from insulin resistance) in whites and Mexican Americans, but not in non-Hispanic blacks.20 Low dairy intake has been positively associated with metabolic syndrome, with risk decreasing as calcium and vitamin D intake increases.6 While these studies only show relationships, the evidence suggests that taking calcium and vitamin D together may help prevent the onset of type 2 diabetes in at-risk populations.19
Falls
Several studies have examined the effect of vitamin D supplementation on falls, which is the leading cause of death from an injury among the elderly. Although vitamin D with calcium is linked to bone density, vitamin D has also been found to help improve muscle mass.3,8,21 Elderly women given vitamin D showed increases in the number and size of type II muscle fibers within 8 to 12 weeks of initiating supplementation.8,21 The reduction in risk of falls through vitamin D supplementation may be as great as 22%.8 A stratified analysis of vitamin D dose found that more than 800 IU daily significantly reduces falls, whereas less than 400 IU does not provide a significant reduction in falls.1 A study by Prince and colleagues demonstrated a 19% reduction in the risk of falls with ergocalciferol and calcium supplementation for noninstitutionalized women with vitamin D deficiency as compared with a control group taking calcium alone.22 Although studies have been inconsistent in demonstrating a positive effect on falls for any but postmenopausal women given both vitamin D and calcium,3 a recent study found an increase in the risk of falls among older community-dwelling woman taking vitamin D.23 The study randomized 2256 women (≥70 years) considered to be at high risk of fracture to receive a single annual dose of 500,000 IU of cholecalciferol, administered orally each autumn or winter for 3 to 5 years, or to placebo. Those receiving cholecalciferol had a 15% increased risk of falls and a 26% increased risk of fractures compared with those receiving placebo.23 The cause for this remains speculative, and the overall data indicate that the optimum dose should be given more regularly.
Mental Health Issues
The presence of vitamin D receptors in the hippocampus and vitamin D’s antioxidative effects may contribute to the relationship between vitamin D levels and some brain-related issues.24 Vitamin D deficiency is positively associated with an increased risk for Alzheimer’s disease, Parkinson’s disease,25 schizophrenia, and depression.1 In Alzheimer’s disease, lower vitamin D levels correlated to lower scores on the Mini-Mental State Examination.26 Vitamin D may also play a role in seasonal affective disorder, which typically occurs when vitamin D levels are lower.27 Subjects receiving vitamin D supplementation for seasonal affective disorder improved significantly over controls receiving phototherapy, and the observed improvement was in direct proportion to serum levels of 25(OH)D.27
Coronary Artery Disease
A relationship has been demonstrated between lower levels of vitamin D and hypertension, coronary artery calcification, and cardiovascular disease.11 In a study of patients with hypertension who were exposed to UVB radiation three times a week for 3 months, both systolic and diastolic blood pressure were found to be reduced by 6 mm Hg.28 Prospective data from a community-based study suggest that vitamin D deficiency correlates with increased cardiovascular risk beyond that of established cardiovascular risk factors.11 This was particularly evident in patients with hypertension who had 25(OH)D levels of 15 ng/mL, as this group had a twofold increased risk of cardiovascular events. A prospective cohort study examining 25(OH)D and 1,25(OH)2D3 demonstrated that low vitamin D levels were associated with increased risk in all-cause and cardiovascular mortality as compared with patients with higher serum vitamin D levels.9 A recent study suggested a possible mechanism for these observations.29 Low vitamin D levels were associated with an increase in asymmetric dimethylarginine, a marker of endothelial dysfunction, and an increase in C-reactive protein, a marker of inflammation.29
Not all studies on the protective effects of vitamin D are conclusive. A study of 340 black patients with type 2 diabetes demonstrated a negative association between vitamin D and visceral adiposity, but a positive association between vitamin D concentration and carotid artery and aortic calcified plaque, which is predictive of future cardiovascular events.30
Osteoporosis
A low vitamin D level is an established risk factor for osteoporosis.6 Inadequate serum vitamin D concentrations will hinder the active transcellular absorption of calcium.6 This action creates a secondary hyperparathyroid state, which mobilizes calcium from the skeleton, reducing bone mineral density and causing a mineralization defect of the collagen matrix that is laid down by osteoblasts.4 While it seems logical to conclude that adequate vitamin D levels reduce the risk of fractures by preventing this cascade of events, the data are inconsistent.
Two randomized trials of calcium (1000 mg) plus vitamin D (800 IU) supplements did not show a reduction in nonvertebral fractures among older women; however, the authors noted that compliance was in the range of 60% to 63%, and that there was a decrease in the risk of hip fractures among adherent participants.31,32 A meta-regression analysis found a greater reduction in nonvertebral fractures with higher doses and higher achieved 25(OH)D levels.32 Based on this finding, the study investigators concluded that fracture prevention with vitamin D is dose-dependent and that higher doses can reduce nonvertebral fractures by approximately 20% for individuals 65 years and older.32 In another study, vitamin D3 in doses of 300 or 400 IU did not have a significant effect on bone density other than in the femoral neck; however, 700 IU taken with 500 mg to 1200 mg of calcium daily prevented bone loss in the lumbar spine, femoral neck, and total body in white individuals.33
Cancer
Studies in animals and humans have demonstrated that adequate vitamin D levels can impact cancer mortality by reducing cancer incidence and improving survival, which is likely attributable to vitamin D’s immunomodulating effect and its regulation of cell growth and differentiation.34 The Health Professionals Follow-Up Study found a 17% reduction in total cancer incidence, a 29% reduction in total cancer mortality, and a 45% reduction in digestive-system cancer mortality with each 10 mg/dL increase in predicted 25(OH)D level.35 In contrast, NHANES III, which included 16,818 men and women, did not demonstrate a relationship between total cancer mortality and vitamin D levels; however, serum 25(OH)D levels were found to be inversely associated with colorectal cancer mortality, with levels of 80 nmol/L or higher associated with a 72% risk reduction compared with levels of 50 nmol/L or lower.36
It appears that certain cancers are more responsive to vitamin D and that a greater benefit may come with the combination of vitamin D and calcium. A randomized, controlled trial found that the combination of vitamin D and calcium in postmenopausal women reduced cancer risk after the first year of treatment.37 While a study involving 3010 women in the Ontario Cancer Registry did not find a significant correlation between dietary vitamin D and calcium intake and breast cancer risk, it did find that use of vitamin D supplements at a dose of 400 IU or more significantly reduced risk.38 The study investigators did not measure serum levels of 25(OH)D, but did control for sunlight exposure. Epidemiologic studies indicate a 30% to 50% increase in the risk of colon, prostate, and breast cancer, as well as an increased risk of mortality from these cancers, when 25(OH)D is below 20 ng/mL.39
In a study of US cancer mortality rates between 1970 and 1994, increased solar UVB radiation correlated with a reduction in non-Hodgkin’s lymphoma and breast, colon, ovarian, and prostate cancer, whereas bladder, esophageal, kidney, lung, pancreatic, rectal, stomach, and corpus uteri cancers demonstrated a higher prevalence with increased solar UVB exposure.40 The authors concluded that careful exposure to solar UVB radiation or, more safely, vitamin D3 supplementation, could extend longevity in many individuals.40 Luscombe and colleagues reported similar findings in men with prostate cancer, noting that those who worked outdoors developed prostate cancer 3 to 5 years later than those working indoors.41
One theory on how vitamin D fights cancer is that 1,25(OH)2D3 induces apoptosis of cancer cells and prevents angiogenesis. In addition, 1,25(OH)2D3 may stimulate the CYP24 gene to produce inactive calcitroic acid, preventing calcium metabolism from being influenced.1 However, in the cancer models, several of the beneficial effects of vitamin D require higher concentrations of 25(OH)D than can be physiologically achieved in humans.37
Multiple Sclerosis
Exposure to the sun in early childhood is associated with a reduced risk of developing multiple sclerosis.42 A case-control study found a 19% reduction in the odds of developing multiple sclerosis among women for every 4.0 ng/mL increase in serum 25(OH)D level.43 Another study of 12 patients taking 40,000 IU of vitamin D for 28 weeks showed a decline in the number of gadolinium-enhancing lesions on magnetic resonance imaging.44 In white patients, the risk of multiple sclerosis decreased by 41% for every increase of 20 ng/mL of 25(OH)D above 24 ng/mL.45
Pain
In observational studies of chronic pain, vitamin D deficiency has been related to impaired neuromuscular functioning, although data to support this are mixed. Several studies have found no link between serum 25(OH)D levels and chronic pain, whereas others have estimated that fibromyalgia may be related to vitamin D deficiency in 40% to 60% of patients.6 At one Minnesota hospital, 90% of 150 patients between the ages of 10 and 65 years who reported nonspecific muscle or bone pain were found to be deficient in vitamin D.4
Chronic Lung Disease
Asthma and chronic obstructive pulmonary disease (COPD) have been associated with the immune and genetic influence of vitamin D.46 Vitamin D deficiency increases the risk of respiratory infections,47 and since exacerbations of COPD often follow these types of infections, higher vitamin D levels may reduce the frequency of acute exacerbations.48 Patients with tuberculosis who were given vitamin D following the sixth week of standard treatment demonstrated improvement on follow-up chest radiographs.48
Measurement and Deficiency
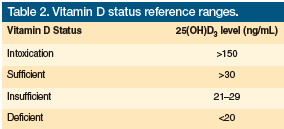
Calcitriol or 1,25(OH)2D3, which is manufactured by the kidneys even when there are significantly low levels of vitamin D, is the active form and is rarely measured in clinical practice. Instead, its precursor, calcidiol or 25(OH)D3, is most commonly measured.49,50 Table 2 outlines vitamin D reference ranges. The cost of measuring vitamin D levels is between $35 and $75 per test.
 When faced with a patient with a low vitamin D level, one must consider whether the deficiency is caused by decreases in absorption, manufacture, or intake of vitamin D.1,49 Because vitamin D is fat-soluble, any disease process or drug that causes fat malabsorption may decrease vitamin D levels.51 Anticonvulsants, glucocorticoids, and certain antiretroviral treatments increase the destruction of vitamin D. Surgery that shortens the small bowel, gastric bypass surgery, Crohn’s disease, celiac disease, or cystic fibrosis may impact vitamin D levels. In obese patients, increased sequestration of vitamin D in fat stores decreases its bioavailability. With significant liver failure, there is a decreased ability to produce 25(OH)D from vitamin D2 or D3. In patients with renal failure, the ability to produce 1,25(OH)2D3 from 25(OH)D decreases. With the nephrotic syndrome, there is a significant urinary loss of 25(OH)D. Studies have shown that there may be a genetic predisposition to vitamin D insufficiency, and genetic variants near genes involved in cholesterol synthesis and vitamin D transport have been found to adversely affect vitamin D levels.52
When faced with a patient with a low vitamin D level, one must consider whether the deficiency is caused by decreases in absorption, manufacture, or intake of vitamin D.1,49 Because vitamin D is fat-soluble, any disease process or drug that causes fat malabsorption may decrease vitamin D levels.51 Anticonvulsants, glucocorticoids, and certain antiretroviral treatments increase the destruction of vitamin D. Surgery that shortens the small bowel, gastric bypass surgery, Crohn’s disease, celiac disease, or cystic fibrosis may impact vitamin D levels. In obese patients, increased sequestration of vitamin D in fat stores decreases its bioavailability. With significant liver failure, there is a decreased ability to produce 25(OH)D from vitamin D2 or D3. In patients with renal failure, the ability to produce 1,25(OH)2D3 from 25(OH)D decreases. With the nephrotic syndrome, there is a significant urinary loss of 25(OH)D. Studies have shown that there may be a genetic predisposition to vitamin D insufficiency, and genetic variants near genes involved in cholesterol synthesis and vitamin D transport have been found to adversely affect vitamin D levels.52
Because a considerable amount of vitamin D is manufactured by the skin, many factors can affect vitamin D levels. Latitude is an important determinant, as direct sun exposure is necessary for adequate manufacture of vitamin D. With the sun’s rays hitting the Earth at an angle during winter months, little, if any, vitamin D is produced in northern latitudes during this time of year, especially in regions above 35 degrees North latitude (eg, Dallas, TX; Atlanta, GA). Sunscreen with a sun protection factor of 15 may decrease skin synthesis of vitamin D by nearly 99%.2 In the elderly, the ability of the skin to manufacture vitamin D may decrease by as much as 75%.2 In addition, with increasing skin pigmentation, the manufacture of vitamin D decreases. A recent study showed that in springtime in a relatively sunny area, sun exposure in elderly African-American women did not cause an increase in their vitamin D levels.53 Even in sunny areas, if the skin is covered, very little vitamin D will be made by the body. Once formed, vitamin D is stored in the body for approximately 3 weeks. Therefore, a seasonal variation in vitamin D levels may occur, as sun exposure can have a considerable effect on serum levels.54
There are currently no evidence-based guidelines to help clinicians determine which patients should have their vitamin D levels tested. Certainly, the diagnosis of vitamin D deficiency or insufficiency should be considered in any individuals with muscle weakness or generalized muscle or bone pain. Testing should also be considered in anyone who has been found to have osteopenia or osteoporosis using bone mineral density measurement. Additionally, the presence of risk factors should help guide clinicians in selecting candidates for testing. Any patient who gets very little sun exposure, has a diet low in dairy or other fortified foods, is on chronic glucocorticoid or seizure medication therapy, has a malabsorption syndrome, or is significantly obese should be considered for vitamin D level testing. The prevalence of vitamin D deficiency in outpatients who are 49 to 83 years of age has been estimated to be 41%.49 At least half of those residing in nursing homes have been found to be either deficient or insufficient in vitamin D.55 An argument could be made for checking the vitamin D levels of most nursing home residents, especially those with muscle weakness or those at a high risk of falls.
Treatment
There are no definitive guidelines on appropriate dosages for vitamin D replacement. According to most studies, maximum benefit from vitamin D replacement is obtained when serum levels are at least 30 ng/mL. Most studies show that fall risk does not decrease unless the dose of vitamin D is at least 700 IU and there is concurrent calcium supplementation.8 It does not make a difference whether the vitamin D comes from dietary sources, supplements, or sunshine. The amount that serum levels will increase is difficult to predict due to differences in absorption and catabolism, but it has been estimated that a daily supplementation of 100 IU of vitamin D3 will raise serum levels by about 1 ng/mL.
For patients with insufficient vitamin D levels (21 ng/mL–29 ng/mL), oral supplementation with vitamin D doses between 1000 IU and 2000 IU is often adequate.1,6 For those who are deficient in vitamin D (levels <21 ng/mL), loading doses of 50,000 IU weekly for 8 weeks is often given, followed by 1000 to 2000 IU daily. It is imperative that vitamin D levels are rechecked in about 3 months due to the wide variability in response to oral supplementation.55 If levels remain lower than 30 ng/mL, the daily dose can be increased or the 50,000 IU weekly dose can be given over a longer period of time.
Direct sun exposure is another way to obtain vitamin D. Directly exposing the arms and legs to the sun between 10:00 am and 3:00 pm at least twice weekly for up to 15 minutes during the summer months will cause enough vitamin D to be produced.1 It has been estimated that getting enough sun exposure to cause minimal erythema while wearing a bathing suit produces about 20,000 IU of vitamin D, whereas sitting in a sunny glassed-in area produces almost no vitamin D, as glass absorbs most of the UVB rays.7
Tanning beds provide UVB radiation, albeit at a lower level than natural sunlight; however, modest exposure to tanning beds can be enough to increase vitamin D to therapeutic levels. This may be a way for those with malabsorption syndromes to maintain vitamin D levels during times when optimal sunlight is not available.56 With UVB exposure, there is concern about increasing the risk of developing nonmelanoma skin cancers, especially basal cell and squamous cell carcinomas.57 While this is certainly a possibility with excessive exposure, 5 to 15 minutes of sun exposure between 10:00 am and 3:00 pm gives only about 25% of the dose of UVB radiation needed to cause a minimal erythemal response, or a slight pinkness to the skin.1 Even a minimal amount of exposure to UVB radiation, depending on latitude, skin pigmentation, and time of year, can allow an adequate amount of vitamin D to be manufactured.
Toxicity
Because vitamin D is a fat-soluble vitamin, there has been concern that excessive oral doses or prolonged sun exposure may cause excessive accumulation; however, large doses of up to 10,000 IU daily for up to 5 months do not cause toxicity.58 This does not preclude the possibility that longer periods of exposure to such doses could cause toxicity; however, there is a mechanism whereby excessive vitamin D made by the skin in response to prolonged sun exposure is destroyed. Some studies suggest an increase in the incidence of nephrolithiasis with large doses of vitamin D, and hypercalcemia or hypercalciuria has also been reported.58
Conclusion
Vitamin D deficiency is a silent epidemic in many parts of the world. With limited dietary intake and limited sun exposure, vitamin D deficiency is a concern in elderly individuals. While the relationship between vitamin D level and bone health has long been known, many lines of evidence now show a link between low vitamin D levels and a higher incidence of cancer, immune deficiencies, multiple sclerosis, cardiovascular disease, and other diseases.9 However, clinicians should keep in mind that the association between lower serum levels of vitamin D and nonskeletal diseases does not prove causation. Controlled clinical studies must be conducted to prove that supplementation with vitamin D will actually prevent these diseases.
It is important to prescribe proper doses of vitamin D. Most studies suggest that 800 IU daily is adequate for maintenance, but much higher doses given consistently may be needed to treat deficiency or insufficiency. Calcium must also be given for maximum benefit. Oral doses of vitamin D may be supplemented by common-sense sun exposure, which may require only 15 minutes of midday sun exposure during the summer months.
The authors report no relevant financial relationships.
Dr. Epplin is clinical professor, Family and Community Medicine, Southern Illinois University School of Medicine, Springfield, and is at Litchfield Family Practice Center, Litchfield, IL; and Sheila A. Thomas is a certified physician assistant and third-year medical student at Oceania University of Medicine, Samoa.
References
1. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
2. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167(16):1730-1737.
3. Brannon PM, Yetley EA, Bailey RL, Picciano MF. Overview of the conference “Vitamin D and Health in the 21st Century: an update.” Am J Clin Nutr. 2008;88(2):438S-490S.
4. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 suppl):1678S-1688S.
5. Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85(3):649-650.
6. Kulie T, Groff A, Redmer J, et al. Vitamin D: an evidence-based review [published correction appears in J Am Board Fam Med. 2010;23(1):138]. J Am Board Fam Med. 2009;22(6):698-706.
7. Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116(8):2062-2072.
8. Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA. 2004;291(16):1999-2006.
9. Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168(12):1340-1349.
10. Brown SJ. The role of vitamin D in multiple sclerosis. Ann Pharmacother. 2006;40(6):1158-1161.
11. Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503-511.
12. Szodoray P, Nakken B, Gaal J, et al. The complex role of vitamin D in autoimmune diseases. Scand J Immunol. 2008;68(3):261-269.
13. Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770-1773.
14. Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79(5):820-825.
15. Danescu LG, Levy S, Levy J. Vitamin D and diabetes mellitus. Endocrine. 2009;35(1):11-17.
16. Hyppönen E, Läärä E, Reunanen A, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358(9292):1500-1503.
17. Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005;48(7):1247-1257.
18. Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017-2029.
19. Pittas AG, Dawson-Hughes, Li T, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29(3):650-656.
20. Scragg R, Sowers M, Bell C; Third National Heart and Nutrition Examination Survey. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2813-2818.
21. Bischoff HA, Stähelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18(2):343-351.
22. Prince RL, Austin N, Devine A, et al. Effects of ergocalciferol added to calcium on the risk of falls in eldery-high risk women. Arch Intern Med. 2008;168(1):103-108.
23. Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial [published correction appears in JAMA. 2010;303(23):2357]. JAMA. 2010;303(18):1815-1822.
24. Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: Preventing “D”ecline? Mol Aspects Med. 2008;29(6):415-422.
25. Evatt ML, Delong MR, Khazai N, et al. Prevalence of vitamin D insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol. 2008;65(10):1348-1352.
26. Oudshoorn C, Mattace-Raso FU, van der Velde N, et al. Higher serum Vitamin D3 levels are associated with better cognitive test performance in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;25(6):539-543.
27. Gloth FM 3rd, Alam W, Hollis B. Vitamin D vs broad spectrum phototherapy in the treatment of seasonal affective disorder. J Nutr Health Aging. 1999;3(1):5-7.
28. Krause R, Bühring M, Hopfenmüller W, et al. Ultraviolet B and blood pressure. Lancet. 1998;352(9129):709-710.
29. Ngo DT, Sverdiov AL, McNeil JJ, Horowitz JD. Does vitamin D modulate asymmetric dimethylarginine and C-reactive protein concentrations? Am J Med. 2010;123(4):335-341.
30. Freedman BI, Wagenknect LE, Hairston KG, et al. Vitamin D, adiposity, and calcified atherosclerotic plaque in African-Americans. J Clin Endocrinol Metab. 2010;95(3):1076-1083.
31. Jackson RD, LaCroix AZ, Gass M, et al; Women’s Health Investigators. Calcium plus vitamin D supplementation and the risk of fractures [published correction appears in N Engl J Med. 2006;354(10):1102]. N Engl J Med. 2006;354(7):669-683.
32. Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169(6):551-561.
33. Cranney A, Weiler HA, O’Donnell S, Puil L. Summary of evidence-based review on vitamin D efficacy and safety in relation to bone health. Am J Clin Nutr. 2008;88(2):513S-519S.
34. Osborne JE, Hutchinson PE. Vitamin D and systemic cancer: Is this relevant to malignant melanoma? Br J Dermatol. 2002;147(2):197-213.
35. Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451-459.
36. Freedman DM, Looker AC, Chang SC, Graubard BI. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2007;99(21):1594-1602.
37. Lappe JM, Travers-Gustafson D, Davies KM, et al. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial [published correction appears in Am J Clin Nutr. 2008;87(3):794]. Am J Clin Nutr. 2007;85(6):1586-1591.
38. Anderson LN, Cotterchio M, Vieth R, Knight JA. Vitamin D and calcium intakes and breast cancer risk in pre- and post-menopausal women. Am J Clin Nutr. 2010;91(6):1699-1707.
39. Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96(2):252-261.
40. Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94(6):1867-1875.
41. Luscombe CJ, Fryer AA, French ME, et al. Exposure to ultraviolet radiation: association with susceptibility and age at presentation with prostate cancer. Lancet. 2001;358(9282):641-642.
42. Kampman MT, Wilsgaard T, Mellgren SI. Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic Circle. J Neurol. 2007;254(4):471-477.
43. Kragt J, van Amerongen B, Killestien J, et al. Higher levels of 25-hydroxyvitamin D are associated with a lower incidence of multiple sclerosis only in women. Mult Scler. 2009;15(1):9-15.
44. Kimball SM, Ursell MR, O’Connor P, Vieth R. Safety of vitamin D3 in adults with multiple sclerosis. Am J Clin Nutr. 2007;86(3):645-651.
45. Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832-2836.
46. Gilbert CR, Arum SM, Smith CM. Vitamin D deficiency and chronic lung disease. Can Respir J. 2009;16(3):73-74.
47. Ginde AA, Mansbach JM, Camargo CA Jr. Vitamin D, respiratory infections, and asthma. Curr Allergy Asthma Rep. 2009;9(1):81-87.
48. Hughes DA, Norton R. Vitamin D and respiratory health. Clin Exp Immunol. 2009;158(1):20-25.
49. Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353-373.
50. Holick MF, Siris ES, Binkley N, et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90(6):3215-3224.
51. Working Group of the Australian and New Zealand Bone and Mineral Society; Endocrine Society of Australia; Osteoporosis Australia. Vitamin D and adult bone health in Australia and New Zealand: a position statement. Med J Aust. 2005;182(6):281-285.
52. Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180-188.
53. Weaver SP, Passmore C, Collins B, Fung E. Vitamin D, sunlight exposure, and bone density in elderly African American females of low socioeconomic status. Fam Med. 2010;42(1):47-51.
54. Peiris AN, Bailey B, Manning T, et al. Testing for vitamin D deficiency in veterans: is there a seasonal bias? J Am Med Dir Assoc. 2010;11(12):128-131.J Am Med Dir Assoc. 2009;10(9):653-657.
56. Koutkia P, Lu Z, Chen T, Holick MF. Treatment of vitamin D deficiency due to Crohn’s disease with tanning bed ultraviolet B radiation. Gastroenterology. 2001;121(6):1485-1488.
57. Barsh G, Attardi LD. A healthy tan? N Engl J Med. 2007;356(21):2208-2210.
58. Vieth R. Why the optimal requirement for vitamin D3 is probably much higher than what is officially recommended for adults. J Steroid Biochem Mol Biol. 2004;89-90(1-5):575-579.










