Alpha-1-glucosidase Inhibitor Prevents Syncope Associated with Postprandial Hypotension
Postprandial hypotension (PPH) is defined as a decrease in systolic blood pressure of 20 mm Hg or more within 2 hours of the start of a meal. Symptoms may include dizziness, lightheadedness, weakness, falls, or syncope.1 Reduction of blood pressure after meals was first described in 1929.2 PPH was documented in a patient with hypertension in 1935, and it was recognized as a clinical problem in a patient with Parkinson’s disease in 1977.3 Over the past two decades, PPH has received special attention by researchers in geriatrics for several reasons. First, PPH among the elderly is fairly common. Several prevalence studies observed that between 24% and 38% of nursing home residents experienced PPH.4-6 Second, disorders of blood pressure regulation, including hypotension, wide pulse pressures, and orthostatic hypotension, are associated with poorer health outcomes and increased mortality in very old people.7-10 Third, PPH occurs more commonly in certain groups of patients who are on dialysis for end-stage renal disease and have preexisting autonomic dysfunction, including those with diabetesmellitus and Parkinson’s disease. Whether through aging or disease, the inability to maintain blood pressure equilibrium indicates both a loss of homeostatic integrity and a vulnerability to falls, which can result in significant injuries.1,11
We report the case of a nondiabetic nursing home resident who experienced symptomatic PPH, including recurrent syncope, despite receiving maximal conventional therapy. To resolve her symptoms, we attempted a novel intervention with miglitol, an alpha-1-glucosidase inhibitor approved by the U.S. Food and Drug Administration (FDA) to treat non–insulin dependent diabetes mellitus. Use of this agent to treat our patient was based on recent human research that showed thatalpha-1-glucosidase inhibitors can also prevent PPH.12-15
Case Presentation
An 84-year-old, nondiabetic, white woman was admitted to a teaching nursing home for subacute rehabilitation following a syncopal episode and fall. Her medical history included failure to thrive, hypertension, coronary artery disease, stroke, hyperlipidemia, gastroesophageal reflux disease (GERD), obstructive sleep apnea, chronic renal insufficiency, hypothyroidism, recurrent Clostridium difficile infection, vascular dementia, depression, and osteoporosis. The patient had no history of diabetes or diabetes treatment. She was dependent on staff for transfers. On admission to the nursing home, her medications included amlodipine and lisinopril for blood pressure, alendronate for osteoporosis, famotidine for GERD, levothyroxine for hypothyroidism, metronidazole for C. difficile infection, escitalopram for depression, and donepezil for dementia. She was also taking aspirin, acetaminophen, and a calcium-vitamin D supplement. Physical examination revealed a thin elderly woman with a seated blood pressure of 130/80 mm Hg and a pulse rate that was regular and within normal limits at 87 beats per minute. She was oriented to person and place. Isolated muscle group testing suggested proximal lower extremity weakness. The patient had non-pitting peripheral edema. Her Saint Louis University Mental Status (SLUMS) Examination score was 11/30 and her Geriatric Depression Scale (GDS) score was 1/15.
The patient’s first hospitalization following admission to the nursing home occurred when she was found unresponsive, hypoxemic, and hypotensive in her room. Hospital evaluation identified no cardiac cause, and the patient was returned to our skilled nursing facility (SNF), where her orthostatic blood pressure was routinely monitored. Blood pressure measurements taken more than 2 hours after meals were not orthostatic. A few days after the patient’s return from the hospital, morning measurements revealed a seated pulse rate of 88 beats per minute, seated blood pressure of 130/76 mm Hg, and a standing blood pressure of 124/69 mm Hg, and her afternoon measurements included a seated pulse rate of 80 beats per minute, a seated blood pressure of 110/79 mm Hg, and a standing blood pressure of 119/79 mm Hg. During physical therapy a few days after these measurements were taken, which took place 2 hours after the patient had eaten breakfast, she reported tiredness upon standing. A therapist documented that the patient exhibited profuse sweating, shortness of breath, and chest discomfort when standing. Pulse and blood pressure measurements were taken and revealed orthostatic hypotension.
New interventions were established, including elevating the head of her bed above 20 degrees at all times to retain baroreceptor tone. Over the weeks that followed, her blood pressure medications were sequentially withdrawn and oral fludrocortisone was added to her medication regimen. To blunt the vasodilator effects of postprandial insulin secretion, the patient was placed on a no concentrated sweets diet and received frequent small feedings. She was also given salt tablets, and compression stockings were placed during waking hours. Despite these interventions, the patient continued to report weakness after meals and was spending an increasing amount of time in bed. She was started on midodrine 2.5 mg orally three times daily with meals, which was titrated up to 5 mg three times daily with meals. While on this regimen, the patient passed out after lunch and was taken to the hospital.
At the hospital, a standard syncope workup was done, including an electrocardiogram, which showed sinus rhythm with occasional premature ventricular beats. Telemetry monitoring was conducted for several days and was unrevealing. An echocardiogram showed normal ventricular function and no valve deformities. Brain imaging, carotid ultrasonography, and electroencephalography were also unrevealing. No tilt table test was performed.
During the patient’s hospitalization, we met with her family several times, and they reported that she had always experienced fatigue after meals and that she had a tendency to faint even before the present series of hospitalizations. Upon close questioning, her condition did not sound like vasodepressor syncope because her son recalled that each time after meals she had reported feeling “woozy” and had wanted to lie down. In the absence of telemetry or rapid response capability, we did not feel that it was safe to perform provocative carotid massage on this patient in the SNF. Therefore, when she was found unresponsive on two occasions during the 1-month period following her admission to the nursing home, while she was receiving aggressive treatment for orthostatic hypotension and PPH, she was sent to the hospital each time for evaluation and returned with no new findings. Routine lunchtime preprandial and postprandial blood pressure measurements were ordered. Two days after her second hospital transfer, she again reported dizziness and weakness after breakfast while receiving bathroom assistance. At that time, her seated blood pressure was 126/86 mm Hg, her seated pulse rate was 110 beats per minute, and her standing blood pressure was 105/86 mm Hg. Her pulse rate while standing was not recorded by the nurse. The patient was placed back in bed.
A few days later, the patient again experienced symptoms after lunch, and blood pressure measurements revealed a supine blood pressure of 134/66 mm Hg, a seated blood pressure of 128/64 mm Hg, a seated pulse rate of 84 beats per minute, and a standing blood pressure of 94/56 mm Hg. Orthostatic pulse rate was not recorded.
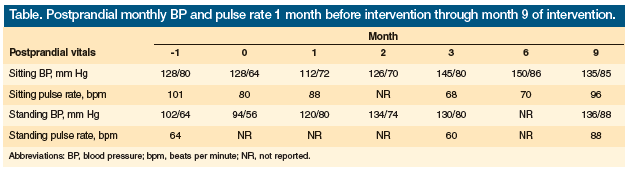
After fully discussing the risks of off-label empiric therapy and the limited clinical evidence available for the use of an alpha-1-glucosidase inhibitor, the patient and her family agreed to a trial of miglitol, which was started at 25 mg orally with each meal. We monitored her blood glucose four times daily. Her glucometer readings were normal, ranging from 91 mg/dL to 165 mg/dL throughout the day. Our patient had no further syncopal or presyncopal episodes after this therapy was started. Her level of alertness and interaction with the staff improved, as did her appetite. After 1 month on miglitol, her postprandial blood pressures had normalized, with a sitting blood pressure of 112/72 mm Hg and a standing blood pressure of 120/80 mm Hg. She had a seated pulse rate of 88 beats per minute. Midodrine and fludrocortisones were tapered. The Table shows the nursing documentation of PPH for the patient 1 month before miglitol therapy was added and up to 9 months thereafter.
Laboratory investigations of orthostasis from the nursing home showed no significant abnormalities in electrolytes, liver function enzymes, blood indices, or endocrine values. Her HgA1c levels indicated no evidence of unmonitored hyperglycemia, and no hypoglycemia was noted on routine glucometer readings.
Discussion
In the nursing home, syncope is multifactorial.16 Our patient’s syncopal episodes likely resulted from her immobility, impaired cerebrovascular autoregulation secondary to atherosclerotic disease, use of antihypertensive drugs, and PPH. The finding that more than one blood pressure disorder affected our patient is consistent with the finding that orthostatic hypotension and PPH coexist in many elderly individuals, and that their effects are additive and not synergistic.17
While both orthostatic hypotension and PPH are prevalent among nursing home residents and increase the risk for falls and syncope in those with impaired cerebrovascular autoregulation due to intrinsic cerebrovascular disease, these blood pressure disorders do not always coexist.18 An observational study showed that elderly patients with syncope had a decrease in their systolic blood pressure after meals, but only half of these individuals had a significant drop in blood pressure consistent with PPH.19 The decline in blood pressure was associated with blood pooling in the splanchnic region without compensatory increases in peripheral vascular resistance, resulting in a decrease in systemic vascular pressure.
It is thought that PPH may result from a direct effect of insulin on arterial dilation, but several studies suggest other pathways and possible therapeutic targets.12-15,20 One target is to decrease gastric motility and slow gastric emptying, which can be achieved with alpha-1-glucosidase inhibitors (Figure).13-15 These agents are available in three formulations: acarbose, miglitol, and voglibose; however, voglibose has not been approved for use in the United States by the FDA. Alpha-1-glucosidase inhibitors stimulate glucagon-like peptide-1 (GLP-1) secretion, which slows gastric emptying and motility, and block glucose absorption at the brush border of the small intestine. Intestinal secretion of potent vasodilator peptide hormones may be directly inhibited independent of glucose mediation.20 This mechanism of action may explain our patient’s marked improvement with an alpha-1-glucosidase inhibitor.
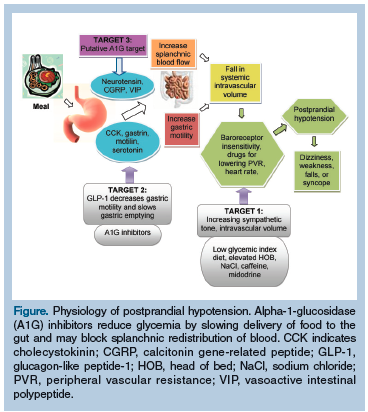
Another target for therapy is the regulation of splanchnic blood flow by gastrointestinal peptide hormonal vasodilators. The action of alpha-1-glucosidase inhibitors on glucose absorption affects a common pathway that influences the secretion of gut hormones with known vasodilator action, such as neurotensin, one of the gastroenteric vasodilator peptides also found in the brain.14,15,20 Studies administering voglibose with meals found that it blunted PPH and diminished secretion of neurotensin. 20,21 While alpha-1-glucosidase inhibitors act on gut peptides and vasodilators with a net effect of decreasing the amount of blood volume diverted to the splanchnic vasculature, their effect on slowing carbohydrate absorption may play an adjunctive role in mediating splanchnic blood flow.22 More research is needed to fully understand why alpha-1-glucosidase inhibitors improve PPH.
Several nonpharmacological interventions also have been investigated for PPH. Among those with some evidence of benefit are changing to a low-carbohydrate diet and consuming caffeinated beverages.1,23 A small study by Vloet and associates found that elderly patients with PPH who received low-carbohydrate meals experienced significantly smaller decreases in systolic blood pressure, shorter duration of PPH, and less PPH-related symptoms compared with those who received normal- or high-carbohydrate meals.24 This may be explained by the postprandial effect of calcitonin gene-related peptide, another vasodilatory neuropeptide produced in peripheral and central neurons, on systemic blood flow.
Miglitol for PPH and Orthostatic Hypertension
A similar case to ours was described by Sasaki and colleagues in a patient with diabetes who presented with PPH and orthostatic dizziness.12 The authors attempted to treat this problem with several medications using three different approaches. First, they sought to increase sympathetic activity with midodrine, an alpha-1 agonist, which decreased the frequency of symptomatic episodes, but did not change the magnitude of the patient’s PPH. Second, they attempted to decrease gastric motility with octreotide, but this did not relieve the patient’s symptoms. Third, they administered acarbose to delay glucose absorption, which reduced the incidence of PPH and its symptoms. Similarly, our patient did not respond to midodrine, but did respond to an alpha-1-glucosidase inhibitor.
Our literature search also found one other study that discusses miglitol as an alternative to acarbose in treating PPH. Arakawa and associates demonstrated that compared with acarbose, miglitol had a greater effect on postprandial hyperglycemia and postprandial insulin requirements.13 Miglitol also had beneficial effects on lipid and interleukin-6 levels.13 Although both alpha-1-glucosidase inhibitors increased the levels of GLP-1, an incretin that delays gastric emptying and blunts PPH, miglitol’s effect was stronger than acarbose at 2 hours after a meal load, with GLP-1 levels of 3.63 pmol/L versus 1.89 pmol/L, respectively (P < .01).13
Conclusion
Our patient experienced debilitating syncopal episodes as a result of her PPH. Once she was treated with miglitol, her symptoms resolved and her quality of life improved, likely due to the effect of miglitol on GLP-1 and secretion of vasodilators in the gut. Our use of miglitol was an off-label, N of 1 trial, and informed consent was obtained and every appropriate measure was taken to ensure patient safety. Our report and others suggest that alpha-1-glucosidase inhibitors should be considered as a novel intervention to treat refractory, symptomatic PPH, and further carefully controlled clinical trials may be warranted, as this class of agents may offer an additional tool for the clinical management of a fairly common geriatric syndrome.
The authors report no relevant financial relationships. Dr. Cruz-Oliver is assistant professor of geriatric medicine, Division of Gerontology and Geriatric Medicine; and Dr. Rodin is associate professor of geriatric medicine, Division of Gerontology and Geriatric Medicine, Saint Louis University School of Medicine, St. Louis, MO.
References
1. Jansen RW, Lipsitz LA. Postprandial hypotension: epidemiology, pathophysiology, and clinical management. Ann Intern Med. 1995;122(4):286-295.
2. Edwards BJ, Perry HM 3rd, Kaiser FE, et al. Relationship of age and calcium generelated peptide to postprandial hypotension. Mech Ageing Dev 1996;87(2):61-73.
3. Seyer-Hansen K. Postprandial hypotension. Br Med J. 1977;2(6097):1262.
4. Vaitkevicius PV, Esserwein DM, Maynard AK, et al. Frequency and importance of postprandial blood pressure reduction in elderly nursing-home patients. Ann Intern Med. 1991;115(11):865-870.
5. Aronow WS, Ahn C. Postprandial hypotension in 499 elderly persons in a long-term health care facility. J Am Geriatr Soc. 1994;42(9):930-932.
6. Fisher AA, Davis MW, Srikusalanukul W, Bude MM. Postprandial hypotension predicts all-cause mortality in older low level care residents. J Am Geriatr Soc. 2005;53(8):1313-1320.
7. Guo Z, Viitanen M, Winblad B. Low blood pressure and five-year mortality in a Stockholm cohort of the very old: possible confounding by cognitive impairment and other factors. Am J Public Health. 1997;87(4):623-628.
8. Molander L, Lövheim H, Norman T, et al. Lower systolic blood pressure is associated with greater mortality in people aged 85 and older. J Am Geriatr Soc. 2008;56(10):1853-1859.
9. Oates DJ, Berlowitz DR, Glickman ME, et al. Blood pressure and survival in the oldest old. J Am Geriatr Soc. 2007;55(3):383-388.
10. Aronow WS, Ahn C. Association of postprandial hypotension with incidence of falls, syncope, coronary events, stroke, and total mortality at 29-month follow-up in 499 older nursing home residents. J Am Geriatr Soc. 1997;45(9):1051-1053.
11. Morley JE. Editorial: Postprandial hypotension-the ultimate Big Mac attack. J Gerontol A Biol Sci Med Sci. 2001;56(12):M741-M743.
12. Sasaki E, Goda K, Nagata K, et al. Acarbose improved severe postprandial hypotension in a patient with diabetes mellitus. J Diabetes Complications. 2001;15(3):158-161.
13. Arakawa M, Ebato C, Mita T, et al. Miglitol suppress the postprandial increase in interleukin 6 and enhances active glucagon-like peptide 1 secretion in viscerally obese subjects. Metabolism. 2008;57(9):1299-1306.
14. Lee A. Preventive treatment with acarbose in diabetic reactive hypoglycemia. J Am Med Dir Assoc. 2010;11(5):377-378.
15. Shibao C, Gamboa A, Diedrich A, et al. Acarbose, an alpha-glucosidase inhibitor, attenuates postrandial hypotension in autonomic failure. Hypertension. 2007; 50(1):54-61.
16. Lipsitz LA, Pluchino FC, Wei JY, Rowe JW. Syncope in institutionalized elderly: the impact of multiple pathological conditions and situational stress. J Chronic Dis. 1986;39(8):619-630.
17. Maurer MS, Karmally W, Rivadeneira H, et al. Upright posture and postprandial hypotension in elderly patients. Ann Intern Med. 2000;133(7):533-536.
18. Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med. 2000;108(2):106-111.
19. Jansen RW, Connelly CM, Kelley-Gagnon MM, et al. Postprandial hypotension in elderly patients with unexplained syncope. Arch Intern Med. 1995;155(9):945-952.
20. Maruta T, Komai K, Takamori M, Yamada M. Voglibose inhibits postprandial hypotension in neurologic disorders and elderly people. Neurology. 2006;66(9):1432-1434.
21. Isa K, Tokashiki T, Tana T, et al. A case of hemodynamic brain infarction with postprandial hypotension [in Japanese]. Rinsho Shinkeigaku. 2002;42(10):959-962.
22. Gentilcore D, Vanis L, Rayner CK, et al. Effects of intraduodenal acarbose on blood pressure, heart rate and splanchnic blood flow in healthy older subjects. J Am Geriatr Soc. 2010;58(4):S186.
23. Rakic V, Beilin LJ, Burke V. Effect of coffee and tea drinking on postprandial hypotension in older men and women. Clin Exp Pharmacol Physiol. 1996;23(6-7):559-563.
24. Vloet LC, Mehagnoul-Schipper DJ, Hoefnagels WH, Jansen RW. The influence of low-, normal- and high-carbohydrate meals on blood pressure in elderly patients with postprandial hypotension. J Gerontol A Biol Sci Med Sci. 2001;56(12):M744-M748.










