Urinary Tract Infections in Older Adults Residing in Long-Term Care Facilities
Urinary tract infection (UTI) is the second most frequent infection in long-term care (LTC) facilities and the most common cause of hospitalization for bacterial infection. Identifying UTI in LTC patients is challenging because of communication barriers, chronic genitourinary symptoms, and comorbidities. Two major sets of guidelines have been published to assist with the diagnosis of symptomatic UTI, but further research is needed to refine these guidelines. Although treating asymptomatic patients is not recommended, this population is often treated in the LTC setting. Antibiotic use for asymptomatic bacteriuria is associated with recurrent infections with multidrug-resistant (MDR) bacteria and does not change survival or chronic genitourinary symptoms. In residents with symptomatic UTI, treatment is similar to that in other ambulatory older adults.
A diagnosis of symptomatic bacterial UTI requires the presence of localizing genitourinary symptoms with significant colony counts of bacteria in the patient’s urine. The definition of significant bacteriuria varies by the method used for collecting the urine sample, the LTC resident’s sex, and whether an indwelling urinary catheter (IUC) is in use. In a midstream urine specimen, the growth of ≥100,000 colony-forming units per milliliter (CFU/mL) of a single bacterial species is diagnostic of bacteriuria (one specimen for men and two consecutive specimens for women).1,2 In a catheterized specimen, the growth of ≥100 CFU/mL of one or more bacterial species is diagnostic.3 Pyuria is the result of inflammation of the genitourinary tract and is detected by more than10 white blood cells per high-power field on microscopic examination of unspun urine.4 It is present in nearly all LTC residents who have bacteriuria, independent of symptoms. Therefore, the presence of pyuria does not signify symptomatic UTI. When bacteriuria is not accompanied by symptoms, it is termed asymptomatic bacteriuria.
Epidemiology
One-quarter of the population of older adults in the United States will reside in an LTC facility at some point in their lives. In this population, UTI is the second most common infection, preceded only by respiratory infection.5 Reports have indicated the prevalence of UTI to range from 0.6% to 21.8%6,7 and its incidence to be between 0.3 and 0.8 cases per 1000 resident care days.8 UTI treatment represents 30% to 50% of antibiotic use in LTC facilities.9 This large range can be explained by the different diagnostic criteria in use, the case mix of patients in each facility, and the high rate of inappropriate use of antibiotics. The rate of IUC use for managing chronic voiding dysfunction in LTC residents ranges from 7% to 10%.3,5 IUCs increase the risk for UTI (relative risk [RR], 2.6), hospitalizations, antibiotic resistance, and death.5,10 The point prevalence of catheter-associated UTI was 5.5% in one study, as compared with 1.1% for those residents without an IUC.7 The incidence was as high as 7.43 per 1000 resident care days in another study.8
In LTC facilities, the prevalence of asymptomatic bacteriuria is higher than that of symptomatic UTI. In noncatheterized residents, asymptomatic bacteriuria has been estimated at 18% to 57% for women and 19% to 38% for men.11 In residents with IUCs, the risk is time-dependent, increasing at a rate of 3% to 8% each day of IUC use, reaching 100% prevalence at 30 days.3
Risk Factors and Pathophysiology
A variety of risk factors predispose LTC residents to developing UTIs. Patient risk factors result from a combination of physiological changes of aging and accumulation of comorbidities. Aging disrupts acquired immunity because of T-cell dysfunction and blunted cytokine-mediated inflammatory response.5 This impaired cellular function is accentuated in the setting of diabetes, cancer, and autoimmune disorders. In addition, comorbidities (eg, dementia, stroke, Parkinson’s disease) result in bladder and bowel incontinence and functional decline, all of which disrupt the body’s innate defense mechanisms.12-14 In women, estrogen deficiency can cause vaginal prolapse and urinary incontinence, promoting an ascending flow of bacteria to the sterile urinary tract. Estrogen deficiency also impairs the protective action of bacterial colonization of the vagina with Lactobacillus, which normally suppresses the growth of pathogenic bacteria.15 In older men, hypertrophy of the prostate causes urinary retention (ie, increased postvoid residual) and turbulent urine flow, predisposing them to chronic prostatitis. The chronically inflamed prostate can form calculi that entrap bacteria, causing recurrent UTIs.16 In contrast to increased postvoid residual in community-dwelling older adults, however, this condition in LTC residents is not associated with an increased rate of UTIs.17,18
In recent years, LTC facilities have admitted residents with higher acuity levels. Consequently, LTC facilities are using more indwelling devices and parenteral antibiotics, exposing a greater number of residents to MDR bacteria.5 Urinary catheterization disrupts patient defense mechanisms, granting bacteria easier access to the bladder. The uropathogens colonize the IUC by binding to host receptors that attach to the surface of the catheter. The bacteria then replicate in the urine that has stagnated near the bulb of the catheter. Bacteria replicating on the IUC have increased virulence; they can create a biofilm by producing exopolysaccharides that entrap and protect replicating bacteria. The biofilm covers the internal and external surface of the catheter and spreads to the bladder in 1 to 3 days. Although the biofilm is produced by only one bacterial species, it promptly becomes polymicrobial in chronic IUC use (>30 days’ duration). These bacteria are highly resistant to antibiotics because they exchange genetic material under the protection of the biofilm, which is impermeable to antibiotics. Catheter encrustations can also obstruct urine flow, promoting urine stagnation and bacterial replication. These encrustations are caused by urease-producing bacteria, which alkalinize the urine, precipitating hydroxyapatite or struvite crystals.3
Microbiology
The types of bacteria isolated and their resistance patterns are different for each LTC facility. In noncatheterized residents and those with short-term IUC placements, a single organism usually causes bacteriuria, although it is polymicrobial in 10% to 20% of cases. In these isolates, Escherichia coli was the most common organism (prevalence, 53%), followed by Proteus (15%) and Klebsiella (14%).19,20 In contrast, bacteriuria is almost always polymicrobial in patients with chronic IUC use. The composition of bacteria in this isolate is dynamic because colonization by a specific organism is transient, with a mean duration of 6 weeks.21 E coli is found in 18% to 35% of cases, Proteus in 10% to 60%, Providencia in 24% to 60%, Enterococcus in 25%, Pseudomonas in 6% to 20%, and Enterobacter in 9%.22
During the last decade, antibiotic resistance in LTC residents has increased significantly. This may be due to the increasing rate of inappropriate use of antibiotics, which has been reported to be as high as 30% to 60%.9,23 In a cross-sectional study of LTC residents seen in the emergency department for UTI, MDR bacteria were identified in 39% to 80% of the isolates, resistance to trimethoprim-sulfamethoxazole in 45% to 86%, and resistance to quinolones in 21% to 61%.24 A cohort of noncatheterized residents in five LTC facilities in Connecticut showed that E coli was resistant to ampicillin 45% of the time, to trimethoprim-sulfamethoxazole 60% of the time, and to quinolones 27% of the time. Proteus, the second most common isolate, was resistant to those antibiotics 22%, 18%, and 36% of the time, respectively.20 Among the risk factors for antimicrobial resistance in LTC residents are recent antibiotic use, diabetes, recent hospitalization, poor functional status, presence of pressure ulcers, use of indwelling catheters, and old age.24,25
Diagnosis
By definition, symptomatic UTI requires the presence of symptoms along with significant bacteriuria. The syndromes of UTI in community-dwelling older adults are well defined and include urethritis (dysuria and hematuria), cystitis (urethritis with urgency, frequency, and suprapubic pain), and pyelonephritis (flank pain, fevers, and nausea/emesis that may or may not be preceded by urethritis/cystitis).1,4 Currently, there is no agreement on an evidenced-based definition for the symptoms of UTI in LTC residents, but it is imperative to distinguish symptomatic UTI from asymptomatic bacteriuria in this population. Treatment of the latter increases the rate of adverse drug effects from the use of antimicrobial medicines; increases the rate of recurrent infections with MDR bacteria; and does not change survival, chronic genitourinary symptoms, or the rate of symptomatic UTI. As a result, the Infectious Diseases Society of America (IDSA) does not recommend treatment of asymptomatic bacteriuria.2
The challenges of diagnosing UTI in LTC residents include communication barriers (dementia and stroke), high prevalence of chronic genitourinary symptoms (incontinence, urgency, frequency, nocturia) at baseline, and lack of a gold-standard laboratory test to confirm clinical suspicion of UTI due to the high prevalence of asymptomatic bacteriuria. Because of the paucity of evidence to support the definition of UTI in LTC facilities, diagnosis during the past three decades has relied on expert consensus recommendations; however, the evidence is weak for each of the signs and symptoms associated with UTI in LTC facilities considered in these recommendations. For example, fever is often considered a symptom, but the definition of fever in LTC facilities has changed since some of the recommendations were made. In 2008, the IDSA defined fever as a single oral temperature of ≥100°F (≥37.8°C), repeated oral temperatures of ≥99°F (≥37.2°C), repeated rectal temperatures of ≥99.5°F (≥37.5°C), or an increase of ≥2°F (≥1.1°C) from the patient’s baseline temperature.26
In 1991, McGeer and colleagues27 issued the first guidelines for managing UTIs in the LTC setting. Their criteria for UTI included a fever of ≥100.4°F (≥38°C) or chills, dysuria, urinary frequency or urgency, flank or suprapubic pain, change in urine character, worsening of mental or functional status, or new or increased incontinence (Table).27-29 These criteria were adopted by the US Department of Health and Human Services and the Centers for Medicare & Medicaid Services as the standard for diagnosis and treatment in LTC facilities. In 2001, the Society for Healthcare Epidemiology of America (SHEA) and the Association for Professionals in Infection Control and Epidemiology issued the second major consensus criteria for antibiotic use in LTC facilities.28 These criteria divided the symptoms into major and minor and strongly encouraged microbiological confirmation.28 In 2005, Loeb and colleagues29 adapted these criteria to guide the treatment of UTI in LTC specifically, and they were applied in a randomized clinical trial to reduce the use of antibiotics to treat UTI in LTC residents. In 2007, a prospective cohort study by Juthani-Mehta and colleagues30 in three community-based LTC facilities in Connecticut demonstrated low accuracy of the McGeer guidelines and the modified Loeb guidelines in predicting bacteriuria and pyuria, which concomitantly constitute laboratory evidence of UTI. The McGeer criteria had a sensitivity of 30%, a specificity of 82%, a positive predictive value of 57%, and a negative predictive value of 61%; the accuracy of the modified Loeb criteria was 30%, 79%, 52%, and 60%, respectively.30 Faced with these findings, the group developed another prospective cohort with 551 LTC residents in the same area who were followed for 1 year. The research group aimed to describe the symptoms associated with laboratory evidence of UTI. After a multivariable analysis adjusted for LTC facilities, age, sex, number of comorbidities, and number of clinically suspected UTI episodes, only dysuria (RR, 51.1-2.0), change in character of urine (RR, 51.1-1.8), and change in mental status (RR, 51.0-1.8) were found to have a significant association.31 The main limitations of this study were low prevalence of dysuria (10%), change in urine character (15.5%), and altered mental status (39.1%) in the cases of suspected UTI; no gold-standard laboratory test (bacteriuria with pyuria is common in asymptomatic LTC residents); and lack of a specific definition for the change in symptoms of urine character and change in mental status, in particular because most of the participants had incontinence and dementia at baseline.
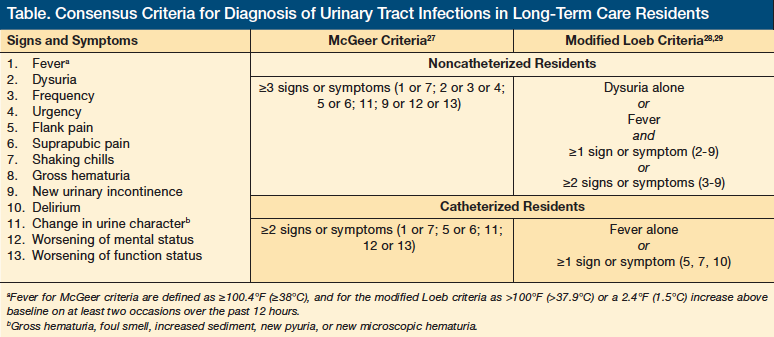
Laboratory Confirmation
Unlike the case for UTI in community-dwelling older adults, a urine specimen with bacteriuria and pyuria in LTC residents is insufficient to confirm a diagnosis of clinically suspected UTI. Both conditions will be present in approximately half of patients without a catheter and in almost all patients with an IUC. Dipstick testing for urine is a fast method for ruling out UTI as the cause of the residents’ symptoms. Juthani-Mehta and colleagues32 demonstrated a negative predictive value of 100% but a positive predictive value of 45% with this method; thus, a positive leukocyte esterase and/or nitrate result is not synonymous with infection, but if both results are negative, the clinician can be certain that there is no UTI.
Rising interest has been shown in alternate methods, such as biomarkers, for diagnosing UTI in LTC residents. Interleukins 8 and 6, lactoferrin, and procalcitonin are used in children and postoperative young adults to identify UTI and to stratify risk.33 The evidence that supports the accuracy of these markers is moderate and research in older adults is warranted. The establishment of a new gold-standard diagnostic test would be useful in clinical practice and for future research involving clinical prediction models that define UTI in LTC facilities.
Management of Symptomatic UTI
The choice of antibiotic to treat UTI in LTC facilities should be individualized on the basis of the patient’s allergy history and renal clearance, local practice patterns, prevalence of resistance at the patient’s LTC facility, availability and cost of the antibiotic, and the threshold for noncompliance. In addition, treatment requires microbiological confirmation and should be tailored toward bacterial susceptibility; empiric treatment can be based on prior urine cultures, if available. The duration of treatment of UTI in noncatheterized LTC residents has not been studied, but SHEA recommends that women with lower UTI (ie, cystitis, urethritis) be treated for 7 days and that women with pyelonephritis and men with any UTI be treated for 10 to 14 days.34 Ruling out coexistent prostatitis in older men is important because this condition requires 6 to 12 weeks of treatment.16 In catheter-associated UTI, the IDSA recommends urine culture and catheter removal or replacement before initiating antibiotics. The early removal of the IUC shortens the duration of febrile episodes and decreases the risk of relapse. The duration of treatment is 10 to 14 days.3
Given the complexity of the diagnosis of UTI in LTC facilities, the increasing resistance to antimicrobials, and the inappropriate use of antibiotics, the use of a facility-adapted protocol to guide diagnosis and antimicrobial selection for suspected UTI is advisable. The best and first example of this practice was the clustered, randomized, controlled trial by Loeb and colleagues29 in 22 LTC facilities in Canada and Idaho that used the aforementioned modified Loeb criteria to reduce the inappropriate use of antibiotics for the treatment of UTI. The intervention also included educational sessions with all members of the healthcare team in the facilities. The LTC facilities in the intervention group used fewer antibiotics to treat UTI than did the usual care homes (weighted mean difference, 0.06-0.93/1000 resident care days); however, neither the total number of antibiotics used for all causes nor the hospitalization and death rates differed between groups.29 These algorithms have been modified and implemented for use in other LTC facilities. Because the prevalence of UTI was higher than the national average in our institution, we implemented a modified diagnostic algorithm designed for nurses to use; this algorithm emphasizes close monitoring and early intervention (Figure). The algorithm separates the symptoms into major and minor based on the likelihood of their association with UTI. It also provides interventions for other common conditions that mimic UTI in LTC residents. Using this algorithm, the rate of suspected UTI decreased by 30% and the rate of antibiotic use for UTI decreased by 20% at 3 months, and these changes persisted at 12 months. Zabarsky and colleagues35 reported similar results using an educational intervention in a Veterans Affairs LTC facility in Ohio.
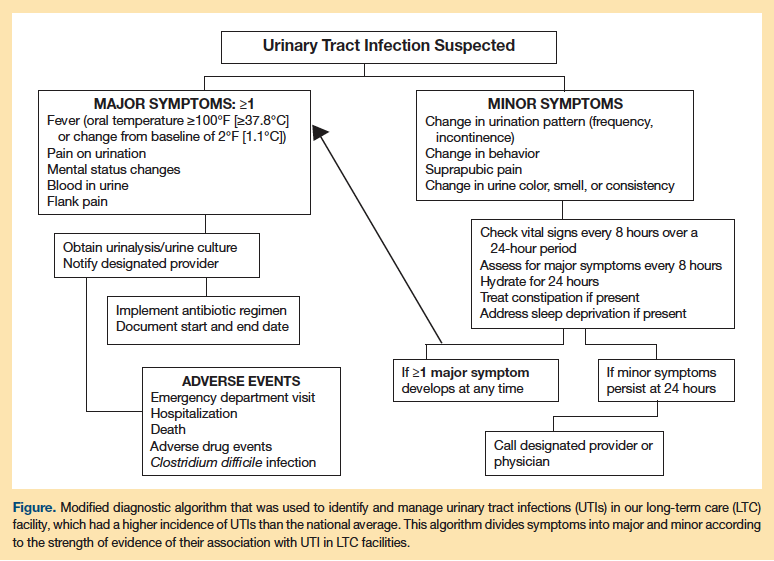
Prognosis
UTI is the most common cause of nursing home–acquired bacteremia, but it has a lower mortality rate than pneumonia. In a New York–based study of older adult LTC residents admitted with nursing home–acquired bacteremia, researchers identified a urinary source in 51% to 56% of cases.36 The mortality rate for residents with nursing home–acquired bacteremia was 8%, as compared with 56% for those with pneumonia.Another observational study in a Veterans Affairs LTC facility in Pittsburgh, Pennsylvania, identified UTI as the cause of nursing home–acquired bacteremia in 55% of cases. The mortality rate for these cases was 15.5%, as compared with a 50% rate for pneumonia cases.37 In a prospective cohort of 13 LTC facilities in California, the death rate was 4% for nursing home–acquired bacteremia and 69% for pneumonia.38 The strongest risk factor for bacteremia is the use of a urinary catheter,independent of the patient’s age, sex, and comorbidities, as well as the type of catheter used.37,39
UTI accounts for one-third of hospitalized patients from LTC facilities. In a retrospective cohort of 4267 LTC facilities in California, Florida, Michigan, New York, and Texas, predictors of hospitalization for UTI were assessed with data from the Minimum Data Set.14 In this cohort, the incidence of hospitalization for UTI was 45.3 cases per 1000 person-years of observation. The risk factors identified were chronic IUC use, Clostridium difficile or MDR infection, comorbidities (ie, Parkinson’s disease, dementia, stroke, diabetes), and polypharmacy (≥10 medications). The researchers found a 69% lower rate of hospitalization in those who could walk independently; further, improving mobility in patients who were impaired reduced their hospitalization rates by 30% to 80%.14 UTI increases the odds of disability (odds ratio, 1.5), and patients with lower function are at a higher risk for UTIs.40
Conclusion
UTI is the most common cause of bacteremia and hospitalization in LTC residents, but its mortality rate is much lower than that for pneumonia. UTI is also the condition for which antibiotics are most frequently prescribed; however, many patients are inappropriately treated. Individuals with asymptomatic bacteriuria should not be prescribed antibiotics, as this practice increases the risk of antimicrobial resistance and does not change chronic genitourinary symptoms or improve survival. Treatment of UTI in LTC residents is similar to that in ambulatory patients, with an emphasis on individualized and tailored antimicrobial therapy. Although two major sets of guidelines have been issued to assist with the diagnosis and treatment of UTIs, these infections remain challenging to manage in LTC residents because of communication barriers, comorbidities, and the presence of chronic urinary symptoms. Further research is needed to shed light on how to optimally diagnose and treat UTIs in the LTC setting.
Funding for this study was provided by NIA Grant T32 AG00029 to Dr. Genao. Dr. Buhr was supported by Geriatric Academic Career Award 1 K01 HP00111-01.
References
1. European Association of Urology. Guidelines on urological infections. 2011. ww.uroweb.org/gls/pdf/15_Urological_Infections.pdf. Accessed April 2, 2012.
2. Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM; Infectious Diseases Society of America; American Society of Nephrology; American Geriatric Society. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults [published correction appears in Clin Infect Dis. 2005;40(10):1556]. Clin Infect Dis. 2005;40(10):643-654.
3. Hooton TM, Bradley SF, Cardenas DD, et al; Infectious Diseases Society of America. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625-663.
4. Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am. 1997;11(3):551-581.
5. Smith PW, Bennett G, Bradley S, et al; Society for Healthcare Epidemiology of America; Association for Professionals in Infection Control and Epidemiology. SHEA/APIC Guideline: Infection prevention and control in the long-term care facility. Am J Infect Control. 2008;36(7):504-535.
6. Nicolle LE, Strausbaugh LJ, Garibaldi RA. Infections and antibiotic resistance in nursing homes. Clin Microbiol Rev. 1996;9(1):1-17.
7. Tsan L, Langberg R, Davis C, et al. Nursing home-associated infections in Department Veteran Affairs community living centers. Am J Infect Control. 2010;38(6):461-466.
8. Stevenson KB, Moore J, Colwell H, Sleeper B. Standardized infection surveillance in long-term care: interfacility comparisons from a regional cohort of facilities. Infect Control Hosp Epidemiol. 2005;26(3):231-238.
9. Loeb M, Brazil K, Lohfeld L, et al. Optimizing antibiotics in residents of nursing homes: protocol of a randomized trial. BMC Health Serv Res. 2002;2(1):17.
10. Kunin CM, Douthitt S, Dancing J, Anderson J, Moeschberger M. The association between the use of urinary catheters and morbidity and mortality among elderly patients in nursing homes. Am J Epidemiol. 1992;135(3):291-301.
11. Nicolle LE; SHEA Long Term Care Committee. Urinary tract infections in long-term care facilities. Infect Control Hosp Epidemiol. 2001;22(3):167-175.
12. Eberle CM, Winsemius D, Garibaldi RA. Risk factors and consequences of bacteriuria in non-catheterized nursing home residents. J Gerontol. 1993;48(6):M266-M271.
13. Powers JS, Billings FT, Behrendt D, Burger MC. Antecedent factors in urinary tract infections among nursing home patients. South Med J. 1988;81(6):734-735.
14. Rogers MA, Fries BE, Kaufman SR, et al. Mobility and other predictors of hospitalization for urinary tract infection: a retrospective cohort study. BMC Geriatr. 2008;8:31.
15. Raz R. Postmenopausal women with recurrent UTI. Int J Antimicrob Agents. 2001; 17(4):269-271.
16. Lipsky BA. Prostatitis and urinary tract infection in men: what’s new; what’s true? Am J Med. 1999;106(3):327-334.
17. Omli R, Skotnes LH, Mykletun A, Bakke AM, Kuhry E. Residual urine as a risk factor for lower urinary tract infection: a 1-year follow-up study in nursing homes. J Am Geriatr Soc. 2008;56(5):871-874.
18. Barabas G, Mölstad S. No association between elevated post-void residual volume and bacteriuria in residents of nursing homes. Scand J Prim Health Care. 2005;23(1):52-56.
19. Nicolle LE. Urinary tract infections in the elderly. Clin Geriatr Med. 2009;25(3):423-436.
20. Das R, Perrelli E, Towle V, Van Ness PH, Juthani-Mehta M. Antimicrobial susceptibility of bacteria isolated from urine samples obtained from nursing home residents. Infect Control Hosp Epidemiol. 2009;30(11):1116-1119.
21. Warren JW, Tenney JH, Hoopes JM, Muncie HL, Anthony WC. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982;146(6):719-723.
22. Nicolle LE. Catheter-related urinary tract infection. Drugs Aging. 2005;22(8):627-639.
23. Nicolle LE. Resistant pathogens in urinary tract infections. J Am Geriatr Soc. 2002;50(suppl 7):S230-S23.
24. Wright SW, Wrenn KD, Haynes M, Haas DW. Prevalence and risk factors for multidrug resistant uropathogens in ED patients. Am J Emerg Med. 2000;18(2):143-146.
25. Muder RR, Brennen C, Drenning SD, Stout JE, Wagener MM. Multiply antibiotic-resistant gram-negative bacilli in a long-term-care facility: a case-control study of patient risk factors and prior antibiotic use. Infect Control Hosp Epidemiol. 1997;18(12):809-813.
26. High KP, Bradley SF, Gravenstein S, et al; Infectious Diseases Society of America. Clinical practice guideline for the evaluation of fever and infection in older adult residents of long-term care facilities: 2008 update by the Infectious Diseases Society of America. J Am Geriatr Soc. 2009;57(3):375-394.
27. McGeer A, Campbell B, Emori TG, et al. Definitions of infection for surveillance in long-term care facilities. Am J Infect Control. 1991;19(1):1-7.
28. Loeb M, Bentley DW, Bradley S, et al. Development of minimum criteria for the initiation of antibiotics in residents of long-term-care facilities: results of a consensus conference. Infect Control Hosp Epidemiol. 2001;22(2):120-124.
29. Loeb M, Brazil K, Lohfeld L, et al. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. BMJ. 2005;331(7518):669.
30. Juthani-Mehta M, Tinetti M, Perrelli E, Towle V, Quagliarello V. Diagnostic accuracy of criteria for urinary tract infection in a cohort of nursing home residents. J Am Geriatr Soc. 2007;55(7):1072-1077.
31. Juthani-Mehta M, Quagliarello V, Perrelli E, Towle V, Van Ness PH, Tinetti M. Clinical features to identify urinary tract infection in nursing home residents: a cohort study. J Am Geriatr Soc. 2009;57(6):963-970.
32. Juthani-Mehta M, Tinetti M, Perrelli E, Towle V, Quagliarello V. Role of dipstick testing in the evaluation of urinary tract infection in nursing home residents. Infect Control Hosp Epidemiol. 2007;28(7):889-891.
33. Nanda N, Juthani-Mehta M. Novel biomarkers for the diagnosis of urinary tract infections: a systematic review. Biomark Insights. 2009;4:111-121.
34. Nicolle LE; SHEA Long-Term-Care-Committee. Urinary tract infections in long-term-care facilities. Infect Control Hosp Epidemiol. 2001;22(3):167-175.
35. Zabarsky TF, Sethi AK, Donskey CJ. Sustained reduction in inappropriate treatment of asymptomatic bacteriuria in long-term care facility through an educational intervention. Am J Infect Control. 2008;36(7):476-480.
36. Mylotte JM, Tayara A, Goodnough S. Epidemiology of bloodstream infection in nursing home residents: evaluation in a large cohort from multiple homes. Clin Infect Dis. 2002;35(12):1484-1490.
37. Muder RR, Brennen C, Wagener MM, Goetz AM. Bacteremia in a long-term-care facility: a five-year prospective study of 163 consecutive episodes. Clin Infect Dis. 1992;14(3):647-654.
38. Beck-Sague C, Villarino E, Giuliano D, et al. Infectious diseases and death among nursing home residents: results of surveillance in 13 nursing homes. Infect Control Hosp Epidemiol. 1994;15(7):494-496.
39. Rudman D, Hontanosas A, Cohen Z, Mattson DE. Clinical correlates of bacteremia in a Veterans Administration extended care facility. J Am Geriatr Soc. 1988;36(8):726-732.
40. Büla CJ, Ghilardi G, Wietlisbach V, Petignat C, Francioli P. Infections and functional impairment in nursing home residents: a reciprocal relationship. J Am Geriatr Soc. 2004;52(5):700-706.










