Torsades de Pointes After Fluoroquinolone Therapy in an Elderly Patient with Comorbidities
Although the proarrhythmic effects of fluoroquinolones are well documented in the literature, awareness of these effects may be lacking in routine clinical practice. Because life-threatening adverse events are possible with the use of these agents, including torsades de pointes, clinicians prescribing fluoroquinolones should carefully consider the risks versus benefits of these agents, especially when prescribing them to elderly patients, to those who have multiple comorbidities, or to those who are taking multiple medications. The authors report the case of an elderly man who was admitted to their institution secondary to acute pulmonary edema and concomitant bronchopneumonia, for which treatment with a fluoroquinolone was initiated. After an electrocardiogram showed a progressive and significant lengthening of the QT interval, fluoroquinolone treatment was discontinued, but the patient still developed torsades de pointes and required defibrillation. The authors review the drug mechanisms of fluoroquinolones and the patient and clinical risk factors that may lead to drug-induced QT prolongation and torsades de pointes, and they briefly discuss measures that can be taken to prevent these adverse effects. (Annals of Long-Term Care: Clinical Care and Aging. 2011;19[2]:35-39)
_____________________________________________________________________________________________________________________________________________
Fluoroquinolones are synthetic broad-spectrum antibiotics that are primarily used to treat bacterial infections. They can be divided into two groups: older fluoroquinolones (eg, ciprofloxacin, norfloxacin, ofloxacin) and newer fluoroquinolones (eg, gemifloxacin, levofloxacin, moxifloxacin).1 Unlike older fluoroquinolones, newer fluoroquinolones have reliable activity against gram-positive bacteria, including streptococci.1 While fluoroquinolones are generally well tolerated and have similar safety profiles to those of other antimicrobial classes, adverse effects have been reported with their use.2 A rare but life-threatening adverse event that has been observed with fluoroquinolone therapy is torsades de pointes, which has been reported to occur at a rate of approximately 0.2 to 2.7 per million prescriptions, with a variability linked to both the quinolone employed and the study considered.3 Although the proarrhythmic effects of fluoroquinolones have been well documented in the literature, it appears that these effects are often overlooked in clinical practice.2 We report the case of an elderly man with several comorbidities who developed torsades de pointes shortly after receiving intravenous (IV) levofloxacin to treat his bronchopneumonia.
Case Presentation
An 80-year-old man was admitted to our hospital for acute pulmonary edema, which was suspected to have been complicated by septic shock. The patient had hyperpyrexia and a productive cough, which he reported had started several days before his hospital admission. His medical history included angina, for which he underwent percutaneous coronary intervention (PCI) of the first diagonal branch in 2005; type 2 diabetes mellitus; chronic renal insufficiency; and respiratory insufficiency due to tuberculosis.
On admission, the patient had a blood pressure of 80/60 mm Hg and a heart rate of 96 beats per minute. An electrocardiogram (ECG) showed sinus rhythm, a left anterior hemiblock, and a QT interval of 390 ms, with a corrected QT interval (QTc) of 470 ms (Figure 1). Blood gas analysis showed a pH of 7.15 (normal, 7.35-7.45), a PCO2 of 41 mm Hg (normal, 35-45 mm Hg), a PO2 of 65 mm Hg (normal, 80-100 mm Hg), and a bicarbonate of 23.7 mmol/L (normal, 21-28 mmol/L), indicating marked respiratory acidosis. Laboratory studies revealed a hemoglobin of 14 g/dL (normal, 14.0-17.5 g/dL); a white blood cell count of 21,000/μL (normal, 4500-11,000/μL), with 85% neutrophils and 12% lymphocytes; a creatinine of 2.4 mg/dL (normal, 0.62-1.2 mg/dL); and a creatinine clearance of 27 mL/min/1.73 m2 (normal, 75-125 mL/min/1.73 m2). A radiograph of the thorax showed a calcified fibrothorax involving the right upper lobe of the lungs and a right medial basal infiltrate. An echocardiogram showed normal dimension and kinesis of the left and right ventricles, an ejection fraction of 55%, and no valvular flow pathologies.
Treatment was initiated with furosemide (0.5 mg/kg/min and bolus of 125 mg x3), dopamine (5 μ/kg/min), dobutamine (5 μ/kg/min), and heparin (5000 IU bolus and 800 IU/h). After consultation with an infectious diseases specialist, levofloxacin IV (500 mg daily) and piperacillin-tazobactam IV (4.5 g twice daily) were started. In addition, the patient’s metabolic acidosis was corrected, and clopidogrel and acetylsalicylic acid were given orally to prevent blood clots.
Monitoring of serial blood samples showed no significant increases in myocardial cytonecrosis enzyme levels. Over the hours that followed, a constant improvement in the patient’s clinical status was observed. He had a good response to the diuretics and his blood pressure normalized. As a result, treatment with dopamine and dobutamine was progressively suspended.
On hospital day 2, an ECG showed a progressive prolongation of the QT interval to 700 ms and a QTc of 666 ms (prolonged in men, >450 ms; Figure 2). Based on this finding, levofloxacin IV therapy was suspended and treatment with ceftriaxone IV was initiated. Despite discontinuing levofloxacin IV, the patient became pulseless and lost consciousness. Ventricular arrhythmia compatible with torsades de pointes was found and defibrillation with a direct current shock up to 250 J was undertaken. The patient regained his senses and his sinus rhythm was restored.
Before discharge from the hospital, coronary radiography was performed, which confirmed the good results of the PCI performed in 2005 and demonstrated no critical coronary lesions. Throughout his hospital stay, telemetry monitoring demonstrated no new arrhythmic episodes, and over the days preceding his hospital discharge, there was a slow and gradual shortening of the QT interval.

Discussion
Fluoroquinolones are widely used to treat a variety of bacterial infections, including gram-negative and grampositive organisms. Although they are generally well tolerated, serious adverse effects have been reported, including QT prolongation and torsades de pointes.2
Drug Mechanisms Causing QT Prolongation
The capacity of fluoroquinolones to block, in a dose-dependent manner, the channels that regulate the outflow of potassium, particularly the rapid component, has been thought to lengthen the cardiac repolarization period.3,4 Studies have indicated that the radical at position 5 of the fluoroquinolone ring may be responsible for causing QT prolongation,5 as the capacity of these agents to lengthen the QT interval has been shown to vary based on the quinolone assessed. For example, sparfloxacin and grepafloxacin, which have an amino and methyl radical at position 5, respectively, have been associated with a mean QTc prolongation of 14 ms and 11 ms, respectively. In contrast, agents with a proton in this position have been associated with less QTc prolongation: 2 ms for ciprofloxacin; 3 ms for gatifloxacin; and between 5 ms and 6 ms for gemifloxacin, moxifloxacin, and levofloxacin.5 Changes to the radical at position 5 have been made in an effort to improve the activity of fluoroquinolones against gram-positive bacteria6; however, because of the extent of QT prolongation, several fluoroquinolones have been withdrawn from the market, including grepafloxacin worldwide and sparfloxacin in the United States.
A retrospective analysis of case reports and clinical studies found that moxifloxacin has the highest risk of QT prolongation of all currently available fluoroquinolones and that ciprofloxacin has the lowest risk.7 Although the study reported that the frequency of torsades de pointes was lower in patients taking gemifloxacin, levofloxacin, and ofloxacin, than in those taking moxifloxacin, these agents should still be used with caution in individuals with risk factors for QT prolongation.7 Owens reported that it is important for healthcare providers to understand which rare adverse effects have been attributed to some members of the quinolone family so that these agents can be avoided in “susceptible” populations, thereby preventing such adverse events.8 Because our patient’s ECG at admission already demonstrated evidence of mild QT prolongation with a QTc of 470 ms (a QTc above 450 in men is considered to be abnormal9), levofloxacin should have been avoided in this patient.
Patient and Clinical Risk Factors for QT Prolongation
Many nonmodifiable risk factors and clinical scenarios can place a patient at risk of developing QT prolongation and torsades de pointes, including older age, female gender, diabetes, renal insufficiency, an underlying cardiovascular condition (eg, hypertension, left ventricular hypertrophy, paroxysmal atrial tachyarrhythmias, heart failure, valvular heart disease, coronary artery disease), polypharmacy, and a combination of these and other factors. A study of 21 patients with druginduced QT interval prolongation and torsades de pointes found that advanced age (>60 years), female gender, hypertension, and paroxysmal atrial tachyarrhythmias were the most common identifiable preexisting factors leading to QT prolongation.10 Another study of drug-induced torsades de pointes among 25 patients who were 80 years and older found female gender and the presence of structural heart disease to be the most common preexisting factors.11
Our patient’s diabetes and age-related renal insufficiency may have played a role in the development of his torsades de pointes. The risk of renal insufficiency increases with age, as glomerular filtration is reduced by 1 mL/min/1.73 m2 annually after the age of 30 years, even in healthy subjects; thus, an individual who is 80 years old may have his or her creatinine clearance reduced by 40%.12 Because levofloxacin is eliminated mainly by renal excretion, there is a prolonged half-life in patients with renal impairment, and dosage adjustments may be necessary in these patients.13 In addition, our patient’s respiratory insufficiency may have led to myocardial ischemia, which could have exacerbated his arrhythmia, leading to torsades de pointes. Another important risk factor for torsades de pointes is polypharmacy. When multiple medications are used, even those that are not likely to overload the body’s elimination pathways, there may be arrhythmogenic effects. For instance, it has been reported that both dopamine and dobutamine should be avoided in patients who have or are suspected to have QT prolongation syndrome, as these agents can lead to R-on-T phenomenon (premature ventricular complex in the ECG interrupting the T wave of the preceding beat), and, subsequently, serious ventricular arrhythmias.14,15
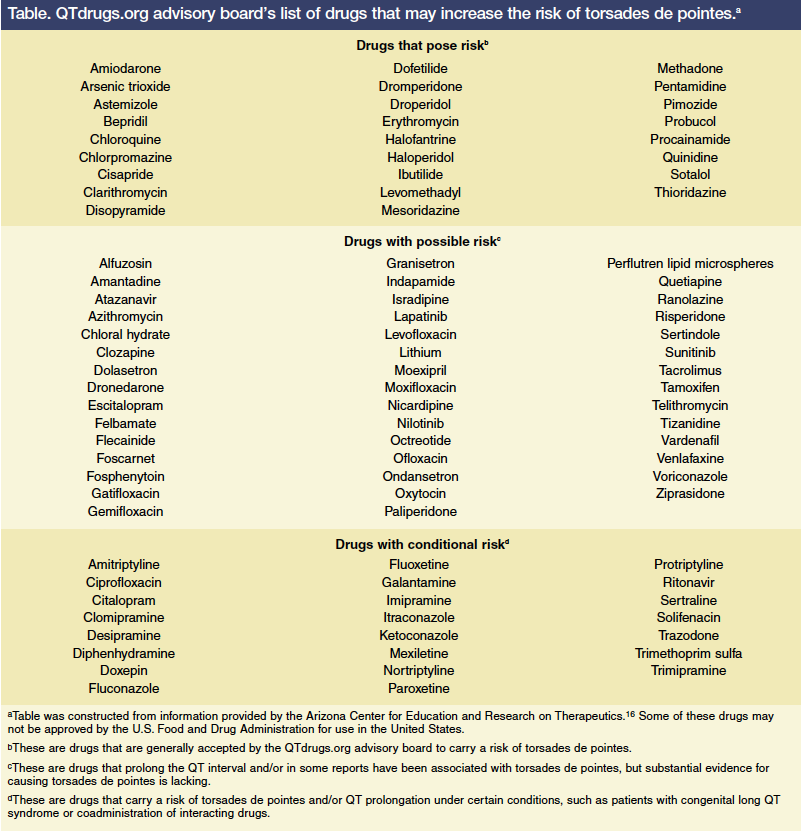
A retrospective review by Paran and colleagues that included 25 patients who were 80 years and older found that administrating QT-prolonging agents despite a long QT interval (n=11; 44%) and coadministration of two or more QT-prolonging agents (n=9; 36%) were the most likely clinical scenarios to lead to drug-induced torsades de pointes.11 Macrolides and quinolones were found to have the greatest effect on the QT interval (n=9; 36%).11 Drugs that may place patients at risk of QT prolongation and torsades de pointes are listed in the Table.16 In another study, cardiac agents (mainly class III antiarrhythmics), antipsychotics, and antibiotics were the medications most commonly found to cause QT prolongation.10 In 5 of 21 cases (24%), potential drug interactions through inhibition of cytochrome P450 isoenzymes were considered to be responsible.10
Our case reminds us of the concept of reduced repolarization reserve, which was introduced by Roden.17 The central idea behind this concept is that every individual has a physiological cardiac repolarization reserve, which is a functional reserve that counterbalances any endogenous (eg, genetic defects, cardiac disorders) or exogenous (eg, pharmacotherapies) factors that would either reduce repolarizing or increase depolarizing currents during the action potential.17 When multiple risk factors for QT prolongation are present, as was the case with our patient, the repolarization mechanisms saturate, resulting in an electrical instability within the ventricle (Figure 3).

Preventing QT Prolongation
Katritsis and Camm suggest that monitoring with ECGs may be necessary following initiation of quinolone therapy in patients who have conditions known to predispose to torsades de pointes or who are receiving concomitant medications that prolong the QT interval.3 However, approximately 3% of all written prescriptions are for drugs that have been shown to lengthen the QT interval or have been associated with torsades de pointes18; thus, identifying preexisting risk factors, assessing renal function before and during treatment, and monitoring high-risk patients with regular ECGs may help minimize occurrences of torsades de pointes. When adverse events occur, including QT prolongation and torsades de pointes, the Naranjo algorithm may help clinicians determine the likelihood that the adverse event was drug-induced, which can facilitate reporting of such events.19
Conclusion
Determining which patients may experience QT prolongation following administration of a fluoroquinolone or another agent poses a considerable challenge in clinical practice, as many factors can work together or independently to disrupt the heart’s electrical system. As our case report demonstrates, it is especially important to consider the potential adverse effects of all treatments, including antibiotics, before prescribing them to patients who are elderly, have comorbidities, are taking multiple medications, or have other risk factors for QT prolongation. If it is determined that the potential benefit of a treatment outweighs the risk, careful patient monitoring is warranted, which may include ECG monitoring and assessments of renal function.
The authors report no relevant financial relationships.
Drs. Proietti, Andrea Rognoni, Maccio, Corrado, and Giorgio Rognoni are consultant cardiologists, Division of Cardiology, Saint Andrew Hospital, Vercelli, Italy.
References
1. The Merck Manuals Online Medical Library. Fluoroquinolones. www.merckmanuals.com/professional/sec14/ch170/ch170f.html. Accessed January 25, 2011.
2. Owens RC Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl 2):S144-S157.
3. Katritsis D, Camm AJ. Quinolones: cardioprotective or cardiotoxic? Pacing Clin Electrophysiol. 2003;26(12):2317-2320.
4. Milberg P, Hilker E, Ramtin S, et al. Proarrhythmia as a class effect of quinolones: increased dispersion of repolarization and triangulation of action potential predict torsades de pointes. J Cardiovasc Electrophysiol. 2007;18(6):647-654.
5. Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother. 2002;49(4):593-596.
6. Tillotson GS. Quinolones: structure-activity relationships and future predictions. J Med Microbiol. 1996;44:320-324. https://jmm.sgmjournals.org/cgi/reprint/44/5/320.pdf.
7. Falagas ME, Rafailidis PI, Rosmarakis ES. Arrhythmias associated with fluoroquinolone therapy. Int J Antimicrob Agents. 2007;29(4):374-379.
8. Owens RC Jr. QT prolongation with antimicrobial agents: understanding the significance. Drugs. 2004;64(10):1091-1124.
9. Medscape Today. QTc prolongation and risk of sudden cardiac death: is the debate over? www.medscape.com/viewarticle/522879. Accessed January 25, 2011.
10. Letsas KP, Efremidis M, Kounas SP, et al. Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol. 2009;98(4):208-212.
11. Paran Y, Mashav N, Henis O, et al. Drug-indiced torsades de pointes in patients aged 80 years or more. Anadolu Kardiyol Derg. 2008;(4)8:260-265.
12. Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41(1):1-12.
13. Traunmüller F, Thalhammer-Scherrerb R, Locker GJ, et al. Single-dose pharmacokinetics of levofloxacin during continuous veno-venous haemofiltration in critically ill patients. J Antimicrob Chemother. 2001;47(2):229-231.
14. David S, Zaks JM. Arrhythmias associated with outpatient dobutamine infusion. Angiology. 1986;37(2):86-91.
15. Kinder C, Chamberlin J, Kail J, et al. Prognostic significance of sustained ventricular tachyarrhythmia occurring during dobutamine infusion. J Heart Lung Transplant. 1994;13(6):1045-1050.
16. Arizona Center for Education and Research on Therapeutics. Torsades lists. Accessed January 31, 2011.
17. Roden DM. Pharmacogenetics and drug-induced arrhythmias. Cardiovasc Res. 2001;50(2):224-231.
18. Gowda RM, Khan IA, Wilbur SL, et al. Torsade de pointes: the clinical considerations. Int J Cardiol. 2004;96(1):1-6.
19. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.










