The Role of Nutrition in Modifiable Geriatric Syndromes
Affiliations: 1 Wellspring Healthcare, Greensboro, NC 2 Friedrich Nutrition Counseling, Salisbury, NC
Article series summary: This is the fifth article in a continuing series on nutrition issues in long-term care. Access the previous articles in this series at www.annalsoflongtermcare.com/category/topics/series-nutrition-issues-long-term-care.
Abstract: The essence of healthcare reform is to shift the paradigm of Western medicine from a treatment model to a prevention model. The inherent benefits of different healthcare interventions for the prevention of disease are debatable. To prioritize the value of different preventive services, the Agency for Healthcare Research and Quality published a technical report titled Common Syndromes in Older Adults Related to Primary and Secondary Prevention, in which they identify eight modifiable syndromes in older adults that frequently lead to institutionalization. The paper focuses on the role of declining nutritional status as a precursor to these syndromes, and outlines nutritional strategies for promoting quality of life in elderly patients. Although the etiology of many of these common geriatric syndromes may not be tied directly to inadequate diet, suboptimal nutrient intake and involuntary weight loss can exacerbate these conditions. Systems that track the effectiveness of nutritional prevention strategies in older adults will facilitate a closer relationship between best practices and the impact of these common syndromes on patient well-being.
Key words: Malnutrition, sarcopenia, frailty, cachexia, vitamin deficiencies, mineral deficiencies.
_________________________________________________________________________________________________________________________________
Many of the leading causes of death and disability in the United States are related to lifestyle-related chronic diseases, such as obesity, diabetes, and hypertension. Three of the common health behaviors that are responsible for the development of many chronic diseases are lack of exercise, poor nutrition, and excessive alcohol intake.1,2 In particular, poor diet and lack of exercise are contributors to conditions that limit ambulation and activities of daily living (ADLs). Often, a decline in health is precipitated by events, such as onset of illness or change in social circumstances, that impact nutrient intake or utilization. Studies have shown that improvements in diet and fitness levels may delay the onset and progression of many of these chronic conditions.3-5
As part of the movement toward proactive preventive healthcare, in 2011, the Agency for Healthcare Research and Quality (AHRQ) commissioned the United States Preventive Services Task Force Technical Expert Panel to create a systematic synthesis of the published evidence on common geriatric syndromes for which prevention strategies could either thwart or alter the course of the syndromes.6 This article examines the role of nutrition in modifying the eight common geriatric syndromes identified in the AHRQ report: multiple morbidities, cognitive impairment, disability, frailty, sarcopenia, malnutrition, homeostenosis, and chronic inflammation.
Prevention Versus Intervention
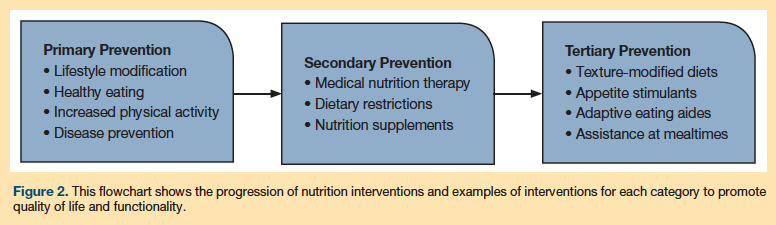
Primary, secondary, and tertiary nutrition-related prevention strategies play critical roles in the medical management of many of these syndromes. As shown in Figure 1, within each category of prevention is an outline of specific interventions. Health prevention that occurs prior to a disease diagnosis is primary; primary nutrition prevention strategies focus on lifestyle modification (eg, healthy eating, patterns of physical activity) and disease prevention. Nutrition-related secondary prevention follows a diagnosis of disease and involves risk reduction and the slowing of disease progression, managed in part with medical nutrition therapy (MNT), with the goal of maintaining function and quality of life. Tertiary prevention aims to slow the progression of disease and reduce suffering; it can also include rehabilitation of disabling conditions. Examples of nutrition-related tertiary prevention interventions include addressing issues related to chewing difficulty or lack of appetite, introducing modified diets, and employing strategies to compensate for restrictions in chewing, swallowing, and self-feeding that may be the result of functional disabilities.7
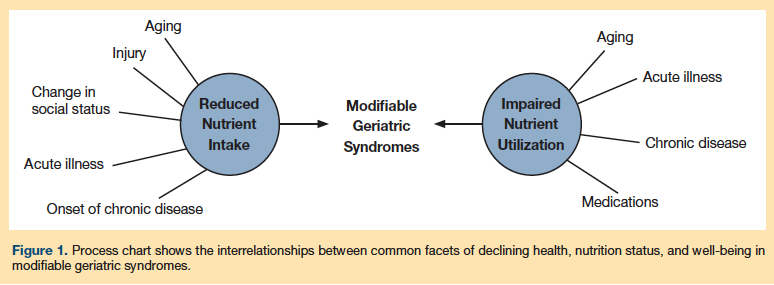
Geriatric Syndromes and Nutritional Status
Geriatric syndromes are complex medical conditions that span multiple physiologic systems and are often related, directly or indirectly, to lifelong health and eating habits. These conditions can lead to age-related decline in general health as well as functional dependency, increased morbidity, and increased risk for institutionalization and mortality.8,9 The AHRQ report examined the prevalence of chronic conditions, high comorbidity scores, polypharmacy, and self-reported poor health in the general elderly population. Many of the top 10 leading causes of death were preventable, as they were related to lifelong dietary habits that could have been managed with MNT.
These syndromes are not independent of one another; many overlap, and it is unclear whether they present concurrently or follow a predictable trajectory of occurrence.6 For example, sarcopenia is associated with frailty and malnutrition.10 Whether the malnutrition precipitates the frailty and sarcopenia, or whether the frailty hastens the malnutrition and sarcopenia is not clear. Other conditions common to this population, such as homeostenosis (decline in homeostatic function) and chronic inflammation, are emerging areas
of research.6
Comprehensive nutritional assessment of adults and the elderly integrates clinical findings for acute and chronic diseases within the context of independence in ADLs. The goal is to provide healthy lifestyle interventions to optimize functional status, health, and well-being, and enhance quality of life. Functional status, as defined by AHRQ in terms of geriatric syndromes, is related to nutritional status in two ways: it is a key factor in overall quality of life for older adults, and it determines whether the individual can meet important nutritional requirements.7 As health and functioning in an individual deteriorate, the types of nutrition and other disciplinary interventions needed will change (Figure 2).7
Nutrition and Multiple Morbidities
More than 20% of older adults suffer from multiple chronic conditions, and prevalence increases with age.6 It has been noted that the majority of adults will be diagnosed with one or more chronic diseases or conditions by age 65 years.11 Older women have a higher prevalence than men of having three or more comorbidities.6 The prevalence of having more than three chronic diseases is higher among African American women (13.4%) compared with white women (9.5%).6 Frailty associated with chronic diseases and inflammatory disorders impacts intermediate activities of daily living (IADL), such as grocery shopping and meal preparation, and can lead to reduced or imbalanced nutritional intake.7 The Women’s Health and Aging Studies I and II demonstrated an increased odds of frailty in women with more than three chronic diseases or more than eight inflammatory conditions.12-14 Specifically, a higher prevalence of frailty has been found in women with nutritional deficits or malnutrition.15
Multiple morbidities usually mean multiple medications. Polypharmacy can often be associated with suboptimal nutrient intake due to changes in taste perception and other side effects that may lead to anorexia. In addition, some medications promote excessive loss of nutrients or impair nutrient utilization.16-18 Successful nutrition prevention strategies to improve the quality of dietary intake while managing nutrients that negatively impact chronic disease management can be instrumental in reducing dosages or the need for selected medications, and for enhancing well-being, quality of life, and functioning.7
Continued on next page
Nutrition and Cognitive Impairment
Cognitive impairment is most commonly described as a subjective complaint of memory loss with objective memory impairment, normal general cognitive function, and intact cognitive ADLs and IADLs.19 The prevalence of cognitive impairment with and without dementia increases with age. The AHRQ report estimates the prevalence of cognitive impairment to be around 30% for all types of dementia, including Alzheimer’s disease (AD), and among community-dwelling adults the prevalence of mild cognitive impairment may exceed 50%.6 Common nutrition-related consequences of declining cognitive function include unplanned weight loss, reduced food intake, and impaired ability to demonstrate IADLs and ADLs, which can, in turn, also lead to impaired nutrition.7,20
The etiology of cognitive impairment may have its roots in a vitamin B12 deficiency, cardiovascular disease, diabetes, hypertension, malnutrition, lifestyle choices, and/or long-term medication use. Nutrition-related cognitive impairment may be preventable through early primary prevention strategies, such as energy- and protein-dense dietary supplements, vitamin mineral supplements, and MNT.6
In one study of 25 nursing home residents with AD followed over 21 consecutive days, 88% of participants were not meeting targeted energy needs, and an estimated 37% prevalence of protein inadequacy was found among the residents.21 Prevention of involuntary weight loss is important because weight loss and low body weight in older adults are predictors of further weight loss,22 morbidity,23 mortality,24,25 and poor quality of life.26 The etiology of weight loss in older adults with AD is unknown, but documented contributors include decline in food intake,21 change in sensory perception of taste and smell,27,28 and impaired ability to regulate dietary intake.29-32 Time of day may also be a factor in total meal intake in elderly residents with dementia, especially those residing in assisted-living or long-term care facilities, as these groups have been shown to experience shifts in circadian eating patterns.21 Among nursing home residents with AD who exhibited behavioral changes, such as increased mental disorganization and confusion, researchers reported the greatest proportion of daily energy was consumed at breakfast, and the greatest deficit in energy intake was associated with diminished eating at dinner.21 In such cases, the provision of high-energy, nutrient-dense foods in the afternoon and evenings would be unlikely to contribute significantly to the individual’s nutrient needs.21
Nutrition-related prevention strategies for residents with cognitive impairment must therefore factor in the individual’s tendencies, incorporating energy- and protein-rich, fortified foods during the mealtimes when he or she is most likely to eat. During mealtimes when the resident is likely to eat less, it can be beneficial to serve nutrient-dense, fortified foods, but in smaller quantities (one-third or half portions), and to offer a substantial snack between meals.
If a resident requires a more restrictive therapeutic diet (eg, low salt, diabetic, renal, low cholesterol33), a more liberal nutrition prevention may be medically prudent. Highly restrictive diets are associated with lower energy intake and therefore may be less effective in managing chronic diseases in older adults, compared with diets with few restrictions.33,34 In these cases, energy-dense nutritional supplements can be a helpful means of hitting energy and protein targets.3-7,35,36 Nutritional supplements can be varied each day based on product and flavor, or blended with pureed fruits, flavored syrups, yogurt, or ice cream to counteract taste fatigue
or monotony.7
Nutrition and Disability
The trajectory toward functional decline is influenced by many factors. Whereas disease and disability (commonly defined as having difficulty with or requiring assistance with ADLs32,37-45) can affect adults of any age, those aged 65 years and older are at the greatest risk for functional decline6 and deterioration in nutritional status.
Minor changes in functioning may seem insignificant to the individual, yet they may represent signs of functional deterioration even without an acute episode of illness or significant change in medical status. Over time, compensatory strategies such as infrequent trips to the grocery store, reduced home inventory of nutrient-dense foods, snacking on low-nutrient items, and skipping meals, lead to suboptimal nutrient intake, food insecurity, and ultimately to institutionalization when care needs can no longer be met independently in the community setting.
Atrophy of skeletal muscle associated with frailty can advance to loss of strength in the muscles of mastication, which can in turn result in a reduced ability to chew whole foods. Moreover, loss of muscles of deglutition (swallowing) may result in a reduced ability to swallow leading to dysphagia. Wasting of the intrinsic muscles of the hand (interosseous and thenar) may result in a decrease in functional efficiency and impair the ability to use eating utensils.46 Loss of hand grip strength is associated with an accelerated dependency in ADLs in adults over age 85 years. Hand grip strength is an objective measure of physical capability that is predictive of frailty, disability,45 and malnutrition.47
The first step in development of nutrition prevention strategies should be identification of potential eating disabilities in the individual. If intakes are suboptimal, one should consider introducing softer textures of foods, adaptive utensils, and assistance at meals as needed. The dining environment should be consistent with the resident’s unique medical needs and any disabilities that may be impairing food intake.3
Nutrition and Frailty
Physical frailty represents a constellation of factors that influence the body’s ability to deal with external stressors (eg, acute illness, falls, other injuries).44 The prevalence of physical frailty in adults 65 years and older is reported to be between 3% and 37% of the population.48 It is characterized by diminished physical strength and endurance, concurrent with involuntary weight loss, which may be due to suboptimal nutrient intake and subsequent loss of fat stores and muscle reserves. Effective medical and nutrition prevention strategies have the potential to prevent and treat physical frailty. Validated screening tools are available for clinicians to identify adults with frailty.49
Effective nutrition interventions for frailty address both fatigue and functional limitations at mealtime. Getting ready for a meal and ambulating to the dining room may trigger crushing fatigue that significantly limits the individual’s ability to eat. Provision of dining locations close to the resident’s room or use of a wheelchair for transport to the dining room can help the individual preserve energy for eating.
The frail resident may find the food portions served to be overwhelming and eat only a few bites at meals. Regular food textures may be too difficult to chew or require too much physical effort. Nutrition interventions for frail residents with small appetites include use of smaller plates, serving only one or two food items at a time, offering small portions of nutrient-dense foods served at times determined by the resident, use of fortified foods, offering protein, and introduction of energy-dense oral nutritional supplements between meals.7,37,38 Texture-modified diets (eg, pureed, liquefied) have been associated with reduced food intake due to limited food choices, less palatable foods, poor presentation, and limitations in palatable between-meal snacks.50 Texture-modified diets should be evaluated for palatability and presentation before being offered. Staff members should taste-test items served on the texture-modified diet and provide input on ways to enhance the taste and presentation of menu items. Collaboration with rehabilitation therapists on the need for adaptive utensils can be helpful. For individuals with fatigue, providing assistance at all meals may be necessary; however, if the individual prefers to self-feed, sufficient time to eat should be provided, and assistance offered as needed.3,7
Nutrition and Sarcopenia
Sarcopenia is a progressive loss of skeletal muscle mass, strength, and function due to disease, medical conditions, sedentary lifestyle, cachexia, or the general aging process. It is not limited to individuals with low body weight. Loss of muscle and reduced hand grip strength are characteristics used for a diagnosis of malnutrition in adults.47 Sarcopenia, on the other hand, is associated with significantly higher likelihood of multiple disabilities, though not mortality, in older adults.6
Lifestyle interventions, including sufficient energy/protein intake and regular physical activity, are vital to thwart sarcopenia. The quality of diet tends to decline in many older adults for a variety of reasons.51-54 Almost 40% of adults aged 70 years and older do not consume the recommended dietary allowance (RDA) for protein (0.8 g/kg/day). Muscle loss in community-living elders was found to be negatively related to protein intake in this population cohort.50 Insufficient intake of high-quality dietary protein may lead to a reduction in lean body mass and increased functional impairment.55 Several researchers now propose raising the RDA for protein intake to 1.2 g.kg/day in older adults.56,57 Low levels of vitamin D also have been found to be associated with incident sarcopenia.58 In older adults, low vitamin D levels may lead to functional limitations including muscle weakness, difficultly rising from a chair, difficulty ascending stairs, and problems with balance.59
Nutrition prevention strategies that address sarcopenia include introducing protein-dense foods at meals the resident is most likely to consume. Use of protein-dense nutritional supplements may be an effective strategy to meet protein requirements. If the muscles of mastication are compromised, a texture-modified diet may be merited (see discussion of ways to enhance a texture-modified diet for better outcomes under the section, Nutrition and Frailty).51 Although the evidence for a benefit in physical performance with vitamin D supplements is controversial,60,61 supplementation that does not exceed the upper tolerability limit may be beneficial.58 Few foods naturally contain vitamin D, and most older adults will require supplementation to maintain sufficient vitamin D stores.7
Continued on next page
Nutrition and Malnutrition
Following the release of the AHRQ report on modifiable geriatric syndromes, the Academy of Nutrition and Dietetics and the American Society for Enteral and Parenteral Nutrition published a consensus paper defining characteristics of adult malnutrition using patient-specific definitions based on etiologies that included social and environmental circumstances and the presence of chronic or acute illness.47 The panel proposed that identification of two or more of the following six characteristics be diagnostic for malnutrition in adults47:
• Insufficient energy intake
• Weight loss
• Loss of muscle mass
• Loss of subcutaneous fat
• Localized or generalized fluid may sometimes mask weight loss
• Diminished functional status as measured by handgrip strength
According to data from the National Health and Nutrition Examination Survey (NHANES, 1999–2000), fewer than one-third of adults aged 60 years and older meet the Healthy Eating Index recommended requirements for consuming meats, dairy, fruits, vegetables, and grains (based on the Food Guide Pyramid).51 More than 80% of participants consumed diets that were categorized as “poor” or “needs improvement.”51,52 Moreover, NHANES data demonstrate a linear relationship between increased age and decreases in total energy, fat, and protein intake in men and women.53,54 Older adults are the most likely segment of the population to become ill from a poor diet.62 In fact, even after moving to an extended care facility, food intake patterns established in those final years at home often persist in the new setting despite an abundance of new food choices due to psychological factors, including depression and confusion.63
In hospitalized elderly patients or those residing in long-term care institutions, as many as 55% and 85%, respectively, are undernourished.64,65 Sullivan65 reported that, after adjustment for other markers of illness severity, patients who were malnourished on admission to a nursing home were at markedly higher risk of life-threatening complications during their stay. Additionally, those who are dependent on feeding assistance are at risk for weight loss. In one Veterans Affairs nursing home, 20% of the total-assist feeders were below average body weight compared with only 10% of the self-feeders or partial-assist feeders.66 Blaum and associates67 found that eating dependency increased the risk of having low body mass index (BMI) or weight loss 1.5- to 2-fold.
As described earlier, many of the eight geriatric syndromes identified in the AHRQ report overlap and/or can contribute to one another’s course. The presence of multiple morbidities,7 frailty,15 polypharmacy,7 cognitive decline/dementia,20,21 and disability68 all can be associated to some degree with poor or malnutrition in older adults. Low energy intake is associated with increased mortality,69 and malnutrition is an independent predictor of mortality in the elderly. Both are associated with involuntary weight loss,70,71 an early indicator of functional decline.72
Nutrition prevention strategies for malnutrition and suspected or confirmed vitamin mineral deficiencies include use of valid screening tools (eg, Mini Nutrition Assessment,73 Malnutrition Screening Tool,74 Malnutrition Universal Screening Tool75), use of guideline-driven MNT, and the introduction of energy- and protein-dense nutritional supplements, vitamin mineral supplements, appetite stimulants, and therapeutic or texture-modified diets.7
Nutrition and Homeostenosis
Homeostenosis is a progressive constriction or “chipping away” of the biological, psychological, and social reserves, thus reducing the body’s ability to adapt to changes and return to homeostasis. All organ systems of the body are affected by homeostenosis. It is a progressive state consistent with the normal trajectory of aging and is not categorized as a disease, nor does it cause immediate disability.76 Associations between homeostenosis and clinical outcomes associated with functional decline, disability, and increased risk for mortality include unstable BMI, unstable pulse pressure, fast plasma glucose, and increased allostatic load.77,78
Few studies have examined the role of impaired homeostasis as a predictor of declining health in older adults. NHANES I and II defined impaired homeostasis in older persons using an allostatic load score to predict the relationship between specific biomarkers and cognitive and physical functioning with aging. In their definition, homeostenosis refers to the state in which the normal physiologic processes wear out or fail to disengage and the body’s internal systems are unable to adapt. The cut points and biomarkers used to measure allostatic load vary across research studies.76,79-81 Some of the biomarkers are recognized to be markers of inflammatory stress (low albumin and low prealbumin; elevated HS-CRP [high sensitivity C-reactive protein], interleukin-6, and fibrinogen). Various methodologies have been proposed to calculate allostatic scores,82 but a standardized criterion has yet to be established.83 Whereas there is limited research on effective interventions to counteract the effects of homeostenosis, the AHRQ concluded that individual studies had demonstrated significant associations between disability, mortality, and signs of homeostatic impairment in the elderly population, and recognized a need for consensus on an operational definition of homeostenosis and its biomarkers in order to better interpret the results of current and future research in this area.6
Nutrition and Chronic Inflammation
Inflammation is characterized by an acute-phase response that triggers a cascade of reactions leading to elevated resting energy expenditure, impaired protein utilization, and increased nitrogen excretion, coupled with anorexia and pathologically altered utilization of nutrients.
Numerous studies have demonstrated that nutritional supplementation alone is not sufficient to reverse the mobilization of nutrients and other cytokine-related changes in organ function that are associated with chronic inflammatory disorders.61 However, adequate nutrient intake is required to support vital organ system function while acute medical treatment is provided. Successful management of an acute episode of illness hinges on both the degree and resolution of the inflammation and whether nutritional requirements continue to be met throughout the treatment process.84,85
Recent evidence suggests that there are varying degrees of acute and chronic inflammation associated with injury, infection, and disease that lead to declining nutritional status and well-being.84-86 This is an emerging area of research that may help clinicians quantify inflammation and determine the effectiveness of different nutrition-related prevention strategies.
Conclusion
The AHRQ report should serve as template for long-term care professionals to incorporate more advanced primary prevention strategies into their practice. The multifactorial etiologies of these common geriatric syndromes warrant an interdisciplinary approach to management and care that considers the overlapping symptoms and contributing comorbidities of each. Although decades of less than optimal lifestyle choices or insufficient primary nutrition prevention strategies may have passed for some long-term care residents, it is never too late to promote healthy lifestyles through secondary and tertiary interventions. Use of primary nutrition prevention strategies and guideline-driven MNT for secondary and tertiary prevention in conjunction with other disciplinary medical or rehabilitative interventions will optimize the health of older adults and ensure that much-needed resources are used most efficiently in the long-term care setting.
References
1. Prevalence of Self-Reported Physically Active Adults—United States, 2007. MMWR Morb Mortal Wekly Rep. www.cdc.gov/mmwr/preview/mmwrhtlm/mm5748a1.htm. 2008;57(48);1297-1300.
2. Behavioral Risk Factor Surveillance System (BRFSS) Prevalence and Trends Data: Atlanta, GA: 2008. Centers for Disease Control and Prevention website. www.cdc.gov/brfss. Accessed August 28, 2014.
3. Bernstein M, Munoz N; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition & Dietetics: food and nutrition for older adults: promoting health and wellness.
J Acad Nutr Diet. 2012;112(8):1255-1277.
4. Simons-Morton DG, Calfas KJ, Oldenburg B, Burton NW. Effects of interventions in healthcare settings on physical activity or cardiorespiratory fitness. Am J Prev Med. 1998;15(4):413-430.
5. Inouye SK, Bogardus ST Jr, Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70-83.
6. Kane RL, Talley KMC, Shamliyan T, Pacala JT. Common Syndromes in Older Adults Related to Primary and Secondary Prevention: Evidence Report/Technology Assessment No. 87. Rockville, MD: Agency for Healthcare Research and Quality; 2011. www.ncbi.nlm.nih.gov/books/n/es87/pdf. Accessed November 13, 2014.
7. Litchford, MD. Common Denominators of Declining Nutritional Status. 3rd ed. Greensboro, NC: CASE Software & Books; 2013:31-38.
8. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):
780-791.
9. Fried LP, Walston JD, Ferrucci L. Part III: Geriatric syndromes: frailty. In: Halter J, Ouslander J, Tinetti ME, Studenski S, High K, Asthana S. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York, NY: McGraw Hill Professional; 2009:631-645.
10. Muhlberg W, Sieber C. Sarcopenia and frailty in geriatric patients: implications for training and prevention. Z Gerontol Geriatr. 2004;37(1):2-8.
11. Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: a 2012 update. Prev Chronic Dis. 2014;11:E62.
12. Szanton SL, Allen JK, Seplaki CL, Bandeen-Roche K, Fried LP. Allostatic load and frailty in the women’s health and aging studies. Biol Res Nurs. 2009;10(3):248-256.
13. Ganguli M, Dodge HH, Mulsant BH. Rates and predictors of mortality in an aging, rural, community-based cohort: the role of depression. Arch Gen Psychiatry. 2002;59(11):1046-1052.
14. Helmer C, Barberger-Gateau P, Letenneur L, Dartigues JF. Subjective health and mortality in French elderly women and men. J Gerontol B Psychol Sci Soc Sci. 1999;54(2):S84-92.
15. Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64(10):1049-1057.
16. Genser, D. Food and drug interaction: consequences for the nutrition/health status. Ann Nutr Metab. 2008;52(suppl 1):29-32.
17. Akamine, D, Filho MK, Peres CM. Drug-nutrient interactions in elderly people. Curr Opin Clin Nutr Metab Care. 2007;10:304-310.
18. Pronsky ZM. Food Medication Interactions. 17th ed. Birchrunville, PA; 2012.
19. Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133-1142.
20. Stewart R, Masaki K, Xue QL, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62(1):55-60.
21. Young KW, Greenwood CE. Shift in diurnal feeding patterns in nursing home residents with Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2001;56(11): M700-M706.
22. Martin CT, Kayser-Jones J, Stotts NA, Porter C, Froelicher ES. Risk for low weight in community-dwelling, older adults. Clin Nurse Spec. 2007;21(4):203-211.
23. Morley JE. Nutrition in the elderly. Curr Opin Gastroenterol. 2002;18(2):240-245.
24. Cronin-Stubbs D, Beckett LA, Scherr PA, et al. Weight loss in people with Alzheimer’s disease: a prospective population based analysis. BMJ. 1997;314(7075):178-179.
25. Nielsen H, Lolk A, Pedersen I, Autzen M, Sennef C, Kragh-Sørensen P. The accuracy of early diagnosis and predictors of death in Alzheimer’s disease and vascular dementia–a follow-up study. Acta Psychiatr Scand. 1991;84(3):277-282.
26. Crogan NL, Pasvogel A. The influence of protein-calorie malnutrition on quality of life in nursing homes. J Gerontol A Biol Sci Med Sci. 2003;58(2):159-164.
27. Wouters-Wesseling W, Wouters AE, Kleijer CN, Bindels JG, de Groot CP, van Staveren WA. Study of the effect of a liquid nutrition supplement on the nutritional status of psycho-geriatric nursing home patients. Eur J Clin Nutr. 2002;56(3):245-251.
28. Carver A, Dobson AM. Effects of dietary supplementation of elderly demented hospital residents. J Hum Nutr Diet. 1995;8(6):389-394.
29. Rolls BJ, Dimeo KA, Shide DJ. Age-related impairments in the regulation of food intake. Am J Clin Nutr. 1995;62(5):923-931.
30. Roberts SB, Fuss P, Heyman MB, et al. Control of food intake in older men. JAMA. 1994;272(20):1601-1606.
31. Moriguti JC, Das SK, Saltzman E, et al. Effects of a 6-week hypocaloric diet on changes in body composition, hunger, and subsequent weight regain in healthy young and older adults. J Gerontol A Biol Sci Med Sci. 2000;55(12): B580-B587.
32. Raji MA, Al Snih S, Ray LA, Patel KV, Markides KS. Cognitive status and incident disability in older Mexican Americans: findings from the Hispanic established population for the epidemiological study of the elderly. Ethn Dis. 2004;14(1):26-31.
33. Tariq SH, Karcic E, Thomas DR, et al. The use of a no-concentrated-sweets diet in the management of type 2 diabetes in nursing homes. J Am Diet Assoc. 2001;101(12):
1463-1466.
34. Taylor KA, Barr SI. Provision of small, frequent meals does not improve energy intake of elderly residents with dysphagia who live in an extended-care facility. J Am Diet Assoc. 2006;106(7):1115-1118.
35. Avenell A, Handoll HH. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev. 2006;18(4):CD001880.
36. Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk for malnutrition. Cochrane Database Syst Rev. 2009;15(2):CD003288.
37. Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295(7):801-808.
38. Tabbarah M, Mihelic A, Crimmins EM. Disability: the demographics of physical functioning and home environments of older Americans. J Archit Plann Res. 2001;18(3):183-193.
39. Chen PC, Wilmoth JM. The effects of residential mobility on ADL and IADL limitations among the very old living in the community. J Gerontol B Psychol Sci Soc Sci. 2004;59(3):S164-S172.
40. Wiener JM, Hanley RJ, Clark R, Van Nostrand JF. Measuring the activities of daily living: comparisons across national surveys. J Gerontol. 1990;45(6):S229-S237.
41. Newman AB, Arnold AM, Sachs MC, et al. Long-term function in an older cohort—the Cardiovascular Health Study All Stars Study. J Am Geriatr Soc. 2009;57(3):432-440.
42. Fuller-Thomson E, Nuru-Jeter A, Minkler M, Guralnik JM. Black-white disparities in disability among older Americans: further untangling the role of race and socioeconomic status. J Aging Health. 2009;21(5):677-698.
43. Fuller-Thomson E, Yu B, Nuru-Jeter A, Guralnik JM, Minkler M. Basic ADL disability and functional limitation rates among older Americans from 2000-2005: the end of the decline? J Gerontol A Biol Sci Med Sci. 2009;64(12):1333-1336.
44. Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009;57(5):830-839.
45. Taekema DG, Gussekloo J, Maier AB, Westendorp RG, de Craen AJ. Hand grip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing. 2010;39(3):331-337.
46. Litchford MD. Nutrition Focused Physical Assessment: Making Clinical Connections. Greensboro, NC: CASE Software & Books; 2012.
47. White JV, Guenter P, Jensen G, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738.
48. Castell M, Sanchez M, Julian R, Queipo R, Martin S, Otero A. Frailty prevalence and slow walking speed in persons age 65 and older: implications for primary care. BMC Fam Prac. 2013;14(86).
49. Morley J. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6).392-397.
50. Wright L, Cotter D, Hickson M, Frost G. Comparison of energy and protein intakes of older people consuming a texture modified diet with a normal hospital diet. J Hum Nutr Diet. 2005;18(3):213-219.
51. Ervin RB. Healthy Eating Index scores among adults, 60 years of age and over, by sociodemographic and health characteristics: United States, 1999-2002. Adv Data. 2008;20(395):1-16.
52. He W, Sengupta M, Velkoff V, Debarros KA. 65+ in the United States: 2005. Current Population Reports – Special Studies. US Census Bureau website. www.census.gov/prod/2006pubs/p23-209.pdf. Published December 2005. Accessed August 29, 2014.
53. Wright JD, Wang CY, Kennedy-Stephenson J, Ervin RB. Dietary intake of ten key nutrients for public health, United States: 1999-2000. Adv Data. 2003;17(334):1-4.
54. Wakimoto P, Block G. Dietary intake, dietary patterns and changes with age: an epidemiological perspective. J Gerontol A Biol Sci Med Sci. 2001;56(2):65-80.
55. Bartali B, Frongillo EA, Bandinelli S, et al. Low nutrient intake is an essential component of frailty in older persons. J Gerontol A Biol Sci Med Sci. 2006;61(6):589-593.
56. Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56(6):M373-M380.
57. Castaneda C, Charnley JM, Evans WJ, Crim MC. Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function and immune response. Am J Clin Nutr.1995;62(1):30-39.
58. Ziambaras K, Dagogo-Jack S. Reversible muscle weakness in patients with vitamin D deficiency. West J Med. 1997;167(6):435-439.
59. Janssen HCJP, Samson MM, Verhaar HJJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002;75(4):611-615.
60. Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2(1):76-89.
61. Lehouck A, Mathiew C, Carremans, C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156(2):105-114.
62. 2008 Older Americans: key indicators of well-being. Federal Interagency Forum on Aging-Related Statistics website. www.agingstats.gov/Main_Site/Data/Data_2008.aspx. Published March 2008, accessed August 29, 2014.
63. Stajkovic S, Aitken EM, Holroyd-Leduc J. Unintentional weight loss in older adults [published correction appears in CMAJ. 2011;183(8):935]. CMAJ. 2011;183(4):443-449.
64. Delano MJ, Moldawer LL. The origins of cachexia in acute and chronic inflammatory diseases. Nutr Clin Pract. 2006;21(1):68-81.
65. Sullivan DH, Morley JE, Johnson LE, et al. The GAIN (Geriatric Anorexia Nutrition) registry: the impact of appetite and weight on mortality in a long-term care population. J Nutr Health Aging. 2002;6(4):275-281.
66. Silver AJ, Morley JE, Strome LS, Jones D, Vickers L. Nutritional status in an academic nursing home. J Am Geriatr Soc. 1988;36(6):487-491.
67. Blaum CS, Fries BE, Fiatarone MA. Factors associated with low body mass index and weight loss in nursing home residents. J Gerontol A Biol Sci Med Sci. 1995;50(3):M162-M168.
68. Cederholm T, Nouvenne A, Ticinesi A, et al. The role of malnutrition in older persons with mobility limitations. Curr Pharm Des. 2014;20(19):3173-3177.
69. Morley JE, Thomas DR. Anorexia and aging: pathophysiology. Nutrition. 1999;15(6):499-503.
70. Verdery RB. Clinical evaluation of failure to thrive in older people. Clin Geriatr Med. 1997;13(4):769-778.
71. Verdery RB. Failure to thrive in old age: follow-up on a workshop. J Gerontol A Biol Sci Med Sci. 1997;52A(6):M333-M336.
72. Robertson RG, Montagnini M. Geriatric failure to thrive. Am Fam Physician. 2004;70(2):343-350.
73. Kaiser MJ, Bauer JM, Ramsch C, et al; MNA-International Group. Validation of the Mini Nutritional Assessment Short-Form (MNA®-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782-788.
74. Ferguson M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition. 1999;15(6):458-464.
75. Todorovic V, Russell C, Elias M, eds. The ‘MUST’ Explanatory Booklet. Redditch, Worcester, UK: BAPEN; 2003. www.bapen.org.uk/pdfs/must/must_explan.pdf. Accessed November 12, 2014.
76. Nelson KM, Reiber G, Kohler T, Boyko EJ. Peripheral arterial disease in a multiethnic national sample: the role of conventional risk factors and allostatic load. Ethn Dis. 2007;17(4):669-675.
77. Zoppini G, Verlato G, Targher G, Bonora E, Trombetta M, Muggeo M. Variability of body weight, pulse pressure and glycaemia strongly predict total mortality in elderly
type 2 diabetic patients: the Verona Diabetes Study. Diabetes Metab Res Rev. 2008;24(8):624-628.
78. Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur Studies of Successful Aging. Proc Natl Acad Sci U S A. 2001;98(8):4770-4775.
79. McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23(5):921-939.
80. Duru OK, Harawa NT, Kermah D, Norris KC. Allostatic load burden and racial disparities in mortality. J Natl Med Assoc. 2012;104(1-2):89-95.
81. Geronimus AT, Hicken M, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826-833.
82. Logan JG, Barksdale DJ. Allostasis and allostatic load: expanding the discourse on stress and cardiovascular disease. J Clin Nurs. 2008;17(7B):201-208.
83. Jensen GL, Bistrian B, Roubenoff R, Heimburger DC. Malnutrition syndromes: a conundrum vs continuum. JPEN J Parenter Enteral Nutr. 2009;33(6):710-716.
84. Jensen GL, Mirtallo J, Compher C, et al. Adult starvation and disease-related malnutrition: a proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. JPEN J Parenter Enteral Nutr. 2010;34(2):156-159.
85. Johnson AM. Low levels of plasma proteins: malnutrition or inflammation? Clin Chem Lab Med. 1999;37(2):91-96.
86. Myron Johnson A, Merlini G, Sheldon J, et al. Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition. Clin Chem Lab Med. 2007;45(3):419-426.
Disclosures: The author has received speaker honoraria from Abbott Nutrition and is a consultant for ProSynthesis Laboratories. The series editor has received speaker honoraria from Abbott Nutrition and has served as a consultant or paid advisory member for Abbott Nutrition.
Address correspondence to: Mary Litchford, PhD, RD, LDN, CASE Software & Books, 5601 Forest Manor Drive, Greensboro, NC 27410; mdlphd@yahoo.com










