Pimavanserin for Treatment of Hallucinations and Delusions Associated With Parkinson Disease Psychosis
In patients with Parkinson disease, psychotic symptoms can be frequent, burdensome, and distressing. The development of pimavanserin represents a novel approach for the treatment of Parkinson disease psychosis (PDP). It is reserved for patients whose hallucinations have not improved after implementing first-line treatment. A review of the pharmacology, pharmacokinetics, clinical trial data, adverse effects, and potential role of pimavanserin in the treatment of hallucinations and delusions associated with PDP was conducted. Findings show that data from ongoing and completed trials indicate pimavanserin is well tolerated, safe, and efficacious. Medications with similar pharmacologic mechanisms are under development and, if successful, may bring a new era of treatment for PDP.
Key words: Parkinson disease, Parkinson disease psychosis, pimavanserin
Parkinson disease (PD) was first medically described in a publication titled An Essay on the Shaking Palsy by James Parkinson in 1817.1 Today, the disease, characterized by involuntary tremulous motions, is recognized as one of the most common neurologic disorders, affecting between 7 to 10 million individuals worldwide.2 Commonly diagnosed in older individuals, the prevalence of PD is projected to increase as the global population ages.3
PD is a debilitating central nervous system (CNS) disorder affecting the motor system due to the death of dopaminergic neurons in the substantia nigra and presence of Lewy bodies. Although no disease-modifying treatments are available for PD, medications aim at either replenishing dopamine (DA) or mimicking the action of DA in the brain. While these dopaminergic medications help to reduce muscle rigidity and tremors and to improve coordination, they can also trigger psychosis characterized by hallucinations and delusions due to the elevation of DA in the brain.4
PD psychosis (PDP) is thought to be the result of 5-HT2A and dopaminergic neuron hyperactivity that is often persistent, progressive, and equally troublesome for the patient.5-8 According to a 12-year study, up to 60% of patients with PD may develop PDP, resulting in a major challenge for treatment and care and, subsequently, an increase in caregiver burden, nursing home admissions, health care cost, and eventually an increase in morbidity and mortality.6,9 PDP is a distressing problem for both patients and their families, acting as a catalyst to move a patient from their home and into a long-term care facility (LTCF). LTCFs then have the difficulty of managing patients with PDP, with limited treatment options to choose from.
Psychotic symptoms are frequent, burdensome, and distressing in patients suffering from PD. Traditional management of PDP consists of initially reducing the dopaminergic agent, then considering add-on therapy for symptom management, such as an atypical antipsychotic.10 The introduction of atypical antipsychotics for the treatment of PDP, however, can further worsen PD motor symptoms through DA antagonism at the D2 receptor. They also lack US Food and Drug Administration (FDA) approval for the treatment of PDP and carry a black box warning of increased morbidity and mortality in elderly patients with dementia and psychosis.3,8
Of the FDA-approved atypical antipsychotics, low-dose quetiapine and clozapine have been most commonly studied in the treatment of PDP. Quetiapine, although well tolerated without significant worsening of motor symptoms, has shown limited efficacy. Four randomized controlled trials, totaling 153 patients with PDP, failed to show efficacy of quetiapine vs placebo.8 One small, double-blind, placebo-controlled trial of 16 patients showed a statistically significant difference on the improved Clinical Global Impression-Severity (CGI-S) for patients randomized to quetiapine vs placebo, showing efficacy and potential clinical benefit in PDP.8,11 A rater-blinded, prospective comparison trial of quetiapine to clozapine for the treatment of PDP in 27 patients found both quetiapine and clozapine provided similar clinical efficacy as indicated by the Clinical Global Impression of Change questionnaire and the Neuropsychiatric Inventory (NPI).12 Clozapine consistently demonstrates efficacy in randomized controlled trials in the treatment of PDP; however, even at low doses, use is significantly limited due to its potentially fatal side effect of agranulocytosis, which requires intensive hematologic monitoring and follow up.3,8 Given that available treatment options thus far have been either unsafe or lack efficacy, development of safe and efficacious alternatives for PDP treatment is a clinical necessity.
Pimavanserin is a selective and potent serotonin 5-HT2A inverse agonist without dopaminergic, adrenergic, histaminergic, or muscarinic affinity.13 This novel atypical antipsychotic, developed by Acadia Pharmaceuticals, was approved on April 29, 2016, and is indicated for the treatment of hallucinations and delusions associated with PDP. This review examines the clinical trial data for pimavanserin and its potential role in therapy for those suffering with PDP.
Pharmacology
In PD, an increase in the number of 5-HT2A receptors may be associated with visual hallucinations due to an increase in 5-HT2A receptor binding in visual processing centers of the brain.6 The importance of 5-HT2A receptors in PDP was established by positronemission tomography, uncovering a direct relationship between visual hallucinations and excessive 5-HT2A neurotransmission.8
Pimavanserin, an inverse agonist at 5-HT2A receptors, acts to a lesser degree at the 5-HT2C receptors and lacks affinity and functional activity at dopaminergic, histaminergic, adrenergic, and muscarinic receptors.7 The development of pimavanserin was based on the similar mechanism of atypical antipsychotics, specifically low-dose clozapine’s ability to act as a potent inverse agonist on 5-HT2A receptor with considerably less potent DA antagonism at the D2 receptor.8 Pimavanserin’s mechanism of action as an inverse agonist is unique. Pimavanserin allows action blockade of 5-HT2A receptor agonists and reverses the activity occurring at the 5-HT2A receptor when no agonist is present. This mechanism is thought to decrease the excessive neurotransmission permitted by the 5-HT2A receptor, ultimately reducing visual hallucinations in PDP.
Pharmacodynamics and Pharmacokinetics
In clinical trials, the drug pimavanserin was studied as pimavanserin tartrate, the salt form. The recommended dose of pimavanserin is 34 mg per day, taken orally as two 17-mg tablets once daily without titration.14 A 34-mg dose of pimavanserin is equivalent to pimavanserin tartrate 40 mg. Pimavanserin can be taken without regard to food. Dosage adjustments are not recommended in patients with mild to moderate renal impairment. Pimavanserin has not been studied in patients with severe renal (CrCl < 30 mL/min) or hepatic impairment; therefore, use is not recommended in these patient populations.4,8
Pimavanserin exhibits potent inverse agonist activity, providing 110% inhibition of the 5-HT2A receptors.4 The median time to maximum serum concentration (Cmax) is roughly 6 hours; the elimination half-life is 57 hours, and the steady-state concentration is estimated to occur after 12 days of therapy. Pimavanserin is highly protein-bound (~95%) and is primarily metabolized by cytochrome P450 (CYP) 3A4 and 3A5 into the active metabolite. Coadministration with moderate to strong CYP3A4 inhibitors results in ~1.5-fold increase in Cmax and ~3-fold increase in pimavanserin’s area under the curve. The recommended dose with strong CYP3A4 inhibitors is 17 mg once daily. Although the administration of pimavanserin with CYP3A4 inducers does not require dose adjustment, monitoring for reduced efficacy is required with possible dose increase depending on clinical efficacy.
Clinical Trials
Essential trials assessing pimavanserin for the treatment of PDP include three placebo-controlled trials and two open-label extension studies.
The first trial (ACP-103-006) was a 4-week, phase 2, randomized, placebo-controlled, double-blind, multicenter study published in 2010.15 A total of 60 patients with L-DOPA or DA-induced hallucinations with or without delusions were randomized to pimavanserin or placebo to primarily assess for safety and tolerability, and secondarily for the effect on PDP. Unified Parkinson’s Disease Rating Scale (UPDRS) Parts II and III (Activities of Daily Living and Motor Examination) were used to assess pimavanserin’s safety on motor function. Compared with placebo, pimavanserin did not show statistically significant improvement in UPRDS II and III (P = .74) or side effects. Efficacy of pimavanserin on symptoms of PDP, primarily evaluated using the Scale for Assessment of Positive Symptoms (SAPS), showed only a trend toward statistical significance in the SAPS total domain score for hallucinations and delusions (P = .09), with statistical significance in improvement in the SAPS global ratings total score of hallucinations and delusions (P = .02). The trial concluded that pimavanserin is safe and well tolerated, does not worsen motor symptoms, and showed some evidence of efficacy in controlling PDP symptoms.15
Based on the safety, tolerability, and efficacy evidence, Acadia designed an international phase 2b/3, multicenter, randomized, placebo-controlled, double-blind trial (ACP-103-012)8 assessing pimavanserin’s efficacy and safety in 298 patients with PDP for 6 weeks. The full results, which have not been published, demonstrated that pimavanserin did not reach statistical significance for the primary outcome of reducing PDP symptoms according to the 20-item SAPS hallucinations and delusions domains (SAPS-H+D) when compared with placebo. The lack of statistical significance was attributed to the large placebo response (42%). Pimavanserin, however, showed safety, motoric tolerability, and suggested benefits in nighttime sleep without daytime sedation and caregiver burden.8,16
A post-hoc analysis of ACP-103-012, though, demonstrated a trend toward statistical significance in US centers where independent centralized raters utilized the validated 9-item SAPS Parkinson’s Disease scale (SAPS-PD) in assessing the primary efficacy endpoint instead of the 20-item SAPS-H+D via live video link. This revealed statistical significance and a clinical advantage of using pimavanserin 40 mg over placebo (P = .05; effect size = 0.44). Use of this methodology provided lower variability and strong separation between pimavanserin and placebo. Additionally, an increase in statistical separation was found in patients with more severe symptoms, defined by NPI score ≥ 6 (P = .022; effect size = .77).3,8,16
Due to the large response rate to placebo, a new study (ACP-103-020)2 was designed with the following changes: (1) inclusion of participants with greater frequency and/or severity of symptoms; (2) use of nonpharmacological psychosocial therapy for 2 weeks to elicit a placebo response ahead of randomization; (3) use of trained, independent raters who were blinded to participants entering or leaving the study, interviewed patients and their caregivers through live video to assess primary efficacy; and (4) use of the 9-item SAPS-PD as the primary efficacy outcome. Higher scores of SAPS-PD reflect an increased severity in PDP and a negative change from baseline score would indicate improvement.3,7 This trial, ACP-103-020, was pimavanserin’s breakthrough trial, facilitating FDA approval for the treatment of PDP. The multicentered study, conducted almost entirely in the United States, was a 6-week, randomized, double-blind, placebo-controlled, phase 3 trial, occurring from August 2010 to August 2012. A total of 198 patients were randomized 1:1 to pimavanserin 40 mg (104 patients) or placebo (94 patients) once daily. After screening patients for inclusion, participants were offered a 2-week period of psychosocial counseling prior to randomization.
The primary outcome of the breakthrough trial was defined as treatment benefit of PDP as assessed by SAPS-PD from baseline to day 43. Pimavanserin demonstrated statistically significant superiority to placebo in decreasing the severity and/or frequency of PDP using the SAPS-PD scale (Table 1) with onset of therapeutic effect seen by day 15 and separation from placebo seen at day 29. With either period in lag time, day 0 through 15 and day 0 through 29, the overlap of concurrent antipsychotics should be avoided due to increased risk of mortality.17
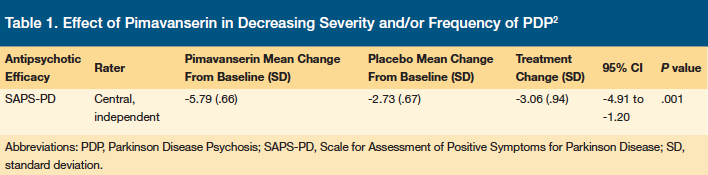
Secondary outcomes (Table 2) included change in CGI-S, Clinical Global Impression-Global Improvement (CGI-I), UPRDS II and III, Zarit Caregiver Burden Scale (CBS), assessment scale of PD nighttime sleep quality (SCOPA-NS) and daytime wakefulness (SOCPA-DS) from baseline to day 43. Pimavanserin was significantly superior to placebo in decreasing CBS, SCOPA-NS, and SCOPA-DS, with small, nonsignificant improvements in motor control (UPDRS II and III) without increasing adverse events (Table 3).
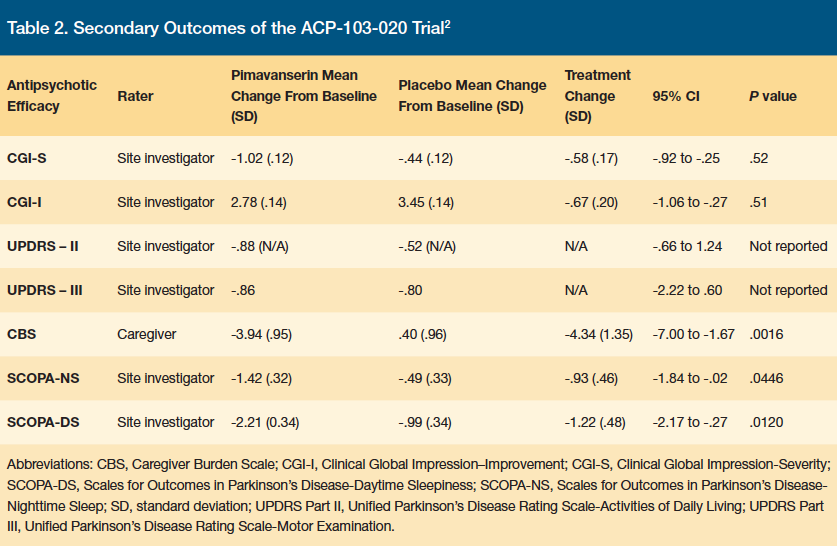
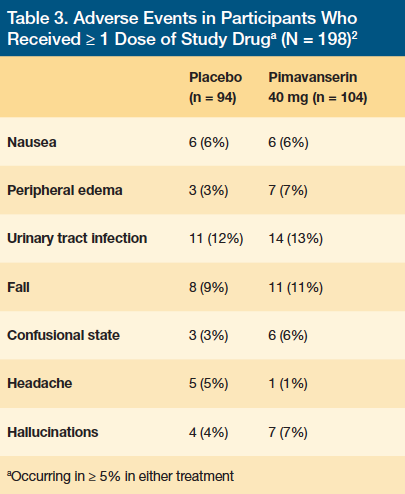
Four (4%) of the patients in the placebo group and 11 (11%) of the patients in the pimavanserin group had a serious adverse event. Two patients in the placebo group and 10 patients in the pimavanserin group discontinued treatment due to an adverse event, with 6 of 10 patients in the pimavanserin group discontinuing due to psychosis. Within 10 days of study drug initiation, 4 of 6 discontinuations occurred prior to patients reaching steady state. Due to pimavanserin’s long half-life of ~57 hours, steady state is not reached until at least day 10 to day 14 of therapy. Although there was an increase in discontinuation in the pimavanserin group compared with placebo, the number of patients dropping out was low compared with other studies involving PDP. Overall, pimavanserin demonstrated efficacy and tolerability in the treatment of PDP at 6 weeks for patients with severe PDP symptoms without significant safety concerns.2
Two open-label extension studies (ACP-103-010 and ACP-103-015) were conducted to determine the long-term efficacy and safety of pimavanserin in treatment of PDP. In both trials, patients remained on pimavanserin for as long as the investigator believed there to be benefit without risk. ACP-103-010 began in November 2004 and ended May 2013. A total of 39 participants from ACP-103-006 trial who could potentially benefit from continuation of pimavanserin were enrolled. Long-term efficacy was measured using CGI-S. Pimavanserin demonstrated continued improvement through week 24, maintaining stabilization through week 72, where ≥ 50% of participants were still actively involved in the study. Further improvement in CGI-S scores was attained in 34% of remaining participants at week 96.16-19
ACP-103-015 began in July 2007, enrolling 459 patients who completed ACP-103-012, -014, or -020 trial and is currently ongoing, with an estimated completion date of December 2017.16,20 Patients previously in the placebo arm were switched to daily pimavanserin. The primary outcome was defined as safety, with the time frame defined by the duration of pimavanserin treatment. Long-term efficacy was measured using SAPS-PD at week 4 of the open-label extension study, in combination with CGI-S, CGI-I, and CBS at all visits.16,20
Evaluation of SAPS-PD at extension week 4 showed that participants previously in the pimavanserin arm maintained SAPS-PD. Participants previously receiving placebo showed improvement in SAPS-PD, similar to the pimavanserin group. Participants previously in the pimavanserin arm demonstrated improved CGI-S, while participants previously receiving placebo showed substantial improvement in CGI-S from week 6 to extension week 24. A similar pattern of minimally to much improved CGI-I scores was also seen, with CBS showing stable benefit over time.18,20 These results suggest potential benefit of pimavanserin beyond 6 weeks for the treatment of PDP.
Pimavanserin demonstrated long-term safety and tolerability, with drug discontinuation due to lack of effectiveness being < 20%. For both studies, a total of 59 patients (11.8%) experienced adverse events. These adverse events were infrequent and were considered scarce or not related to pimavanserin therapy by the investigator.8 However, it is important to note that approximately 65% of patients dropped out of the study by week 96 without any specific reasoning.16
Adverse Events and Safety
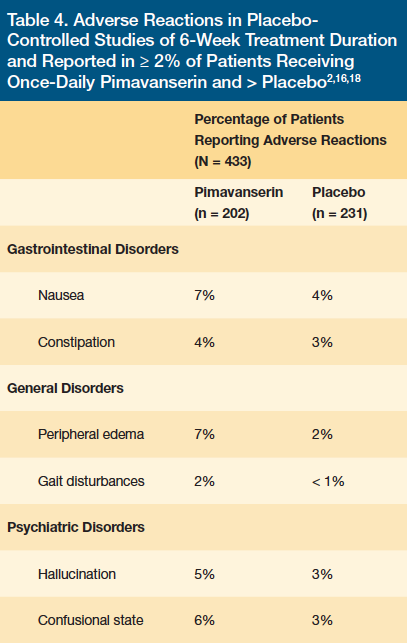
Pimavanserin was well tolerated in all clinical trials discussed above. Statistically significant adverse reactions were defined by an incidence ≥ 5% and at least twice the rate to placebo. These included peripheral edema and confusional state. Other common adverse reactions noted in the study groups compared with placebo were broken down by organ system: gastrointestinal, general, and CNS disorders (Table 4).2 Gastrointestinal reactions included nausea (7% vs 4%) and constipation (4% vs 3%). General disorders included peripheral edema (7% vs 2%) and gait disturbance (2% vs < 1%). Like other antipsychotics, pimavanserin can cause orthostatic hypotension, which can lead to gait disturbance and falls.16 CNS reactions were characterized by hallucination (5% vs 3%) and confusional state (6% vs 3%).2 A total of 8% (16 of 202) of the pimavanserin-treated patients vs 4% (10 of 231) of placebo-treated patients discontinued therapy due to adverse reactions. The adverse reactions that occurred in more than one patient and with an incidence at least twice that of placebo were hallucinations (2% vs 1%), urinary tract infections (1% vs.< 1%), and fatigue (1% vs 0%).18
Two major safety concerns associated with the use of pimavanserin are an increased risk of mortality in elderly patients with dementia-related psychosis and QT prolongation. Antipsychotic drugs increase all-cause risk of death in elderly patients with dementia-related psychosis. Analyses of 17 dementia-related psychosis, placebo-controlled trials revealed a risk of death in drug-treated patients of 1.6 to 1.7 times that of placebo-treated patients.20 Although the causes of death varied, the most common were cardiovascular or infectious in nature. Examining the long-term PDP open-label trial, ACP-103-105, there were 51 deaths among 459 treated patients with PDP (11.1%).21 All deaths, excluding two, were considered unrelated to or unlikely to be associated with pimavanserin as deemed by the treatment providers. Deaths occurring in the pimavanserin group did not appear to be uniquely different than one might expect with the disease course of PDP; however, deaths occurred more frequently in the pimavanserin group vs the placebo group. Therefore, pimavanserin is not approved for the treatment of patients with dementia-related psychosis unrelated to the hallucinations and delusions associated with PDP due to risk of mortality.21 Elders residing in LTC have a higher prevalence of dementia-related psychosis, and health care professionals should understand the significance of increased mortality if pimavanserin is used in the absence of PDP.
Incidence of QT interval prolongation was not defined in the studies above.22 A small but clear increase in QT interval without associated cardiac adverse events was noted in the pimavanserin groups of the studies presented.22 Because the QT interval increased by a mean of 7.3 ms in the pimavanserin group, concomitant use of medications that prolong the QT interval may increase the risk of cardiac arrhythmias, including torsades de pointes and/or sudden cardiac death.2 The package insert recommends avoiding pimavanserin with any concomitant medication known to prolong the QT interval, including class 1A and class 3 anti-arrhythmics, antipsychotics such as ziprasidone, chlorpromazine, thioridazine, as well as fluoroquinolone antibiotics.18 It is also recommended to avoid pimavanserin in patients with a history of cardiac arrhythmias or other cardiac abnormalities.
Conclusion
As a selective 5-HT2A inverse agonist, pimavanserin is the first antipsychotic used in PDP without compromising tolerability or safety like most dopaminergic agents on the market. The data from the clinical trials presented indicate pimavanserin is well tolerated, safe, and efficacious for PDP symptoms.
To date, treatment with pimavanserin has continued in more than 250 participants for over 1 year and more than 150 participants for over 2 years.18,20 The analysis of long-term data has showed continued safety and tolerability following long-term administration of pimavanserin. The development of pimavanserin represents a novel approach in the treatment of PDP and is suited for patients with hallucinations that have not resolved after initial therapy and continue to be bothersome for the patient and/or their family. Specifically, patients with moderate to severe PD treated with pimavanserin have shown improvements in hallucinations and delusions, clinical global impression, caregiver burden, nighttime sleep quality, and daytime wakefulness. Incidence of PDP increases placement of elders into LTCFs, and the benefits of pimavanserin would not only significantly impact elders currently residing in LTCFs but potentially delay elder placement into care facilities. Other medications with a similar pharmacologic mechanism of serotonergic action are under development and, if successful, may bring a completely new era of treatment to PDP.
1 Parkinson J. An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci. 2002;14(2):223-236.
2. Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomized, placebo-controlled phase 3 trial. Lancet. 2014;383(9916):533-540.
3. Hacksell U, Burstein E, McFarland K, et al. On the discovery and development of pimavanserin: a novel drug candidate for Parkinson’s psychosis. Neurochem Res. 2014;39(10):2008-2017.
4. Markham A. Pimavanserin: First global approval. Drugs. 2016;76(10):1053-1057.
5. Friedman JH, Ravina B, Mills R, et al. A multicenter, placebo controlled, double blind trial to examine the safety and efficacy of pimavanserin in the treatment of psychosis in Parkinson’s disease. Neurology. 2010;77:416-421.
6. Ballanger B, Strafella AP, van Eimeren T, et al. Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch Neurol. 2010;67(4):416-421.
7. Bernal-Pacheco O, Limotai N, Go CL, Fernandez HH. Nonmotor manifestations in Parkinson’s disease. Neurologist. 2012;18(1):1-16.
8. Chendo I, Ferreira JJ. Pimavanserin for the treatment of Parkinson’s disease psychosis. Expert Opin Pharmacother. 2016;17(15):2115-2124.
9. Forsaa EB, Larsen JP, Wentzel-Larson T, et al. A 12-year population-based study of psychosis in Parkinson disease. Arch Neurol. 2010;67(8):996-1001.
10. International Parkinson Movement Disorder Society. Update: treatments for non-motor symptoms of parkinson’s disease - December 2012. http://www.movementdisorders.org/MDS-Files1/PDFs/EBM-Papers/EBM-NMS-Updated15Jan2014.pdf. Updated November 3, 2013. Accessed May 23, 2017.
11. Fernandez HH, Okun MS, Rodriguez RL, et al. Quetiapine improves visual hallucinations in Parkinson disease but not through normalization of sleep architecture: results from a double-blind clinical-polysomnography study. Int J Neurosci. 2009;119(12):2196-2205.
12. Merims D, Balas M, Peretz C, Shabtai H, Giladi N. Rater-blinded, prospective comparison: quetiapine versus clozapine for Parkinson’s disease psychosis. Clin Neuropharmacol. 2006;29(6):331-337.
13. Vanover KE, Robbins-Weilert D, Wilbraham DG, et al. Pharmacokinetics, tolerability, and safety of ACP-103 following single or multiple oral dose administration in healthy volunteers. J Clin Pharmacol. 2007;47(6):704-714.
14. Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals Inc; 2016. acadia-pharm.com/wp-content/uploads/2016/04/NUPLAZID-pimavanserin-Package-Insert.pdf. Accessed May 22, 2017.
15. Meltzer HY, Mills R, Revell S, et al. Pimavanserin, a serontonin2A receptor inverse agonist, for the treatment of Parkinson’s disease psychosis. Neuropsychopharmacology. 2010;35(4):881-892.
16. Hermanowicz S, Hermanowicz N. The safety, tolerability and efficacy of pimavanserin tartrate in the treatment of psychosis in Parkinson’s disease. Expert Rev Neurother. 2016;16(6):625-633.
17. Ballard C, Issacson S, Mills R, et al. Impact of current antipsychotic medications on comparative mortality and adverse events in people with Parkinson disease psychosis. J Am Med Dir Assoc. 2015;16(10):898.e1-7.
18. Mills R, Isaacson S, Azulay JP, et al. Long-term effectiveness of NUPLAZID (pimavanserin) in PD psychosis: data from 2 open-label studies. Parkinsonism Relat Disord. 2016;22(suppl 2):e28.
19. An open-label safety study of pimavanserin in Parkinson’s disease patients [clinical trial]. Bethesda, MD: National Library of Medicine (US); April 18, 2017. https://clinicaltrials.gov/ct2/show/NCT01518309. Accessed May 10, 2017.
20. Acadia Pharmaceuticals Inc. NuplazidTM (pimavanserin) sponsor background information for a meeting of the psychopharmacological drugs advisory committee on 29 March 2016. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PsychopharmacologicDrugsAdvisoryCommittee/UCM492453.pdf. Accessed April 19, 2017.
21. Center for Drug Evaluation and Research. FDA Medical Review, NDA 207318, Nuplazid® (pimavanserin). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016 /207318Orig1s000MedR.pdf. Published April 9, 2015. Accessed May 20, 2017.
22. A study of the safety and tolerability of pimavanserin (ACP-103) in patients with parkinson’s disease psychosis. Bethesda, MD: National Library of Medicine (US); May 17, 2016. https://clinicaltrials.gov/ct2/show/NCT00550238. Accessed April 19, 2017.









