Management of Anemia in LTC
Anemia is a common occurrence in long-term care facilities (LTCFs), with prevalence ranging from 34-60%,1,2 while incidence grows substantially with each decade of life after 70 years.1 A condition characterized by a clinically significant reduction in oxygen-carrying capacity of the body, anemia may be driven by a multitude of underlying factors including blood loss, decreased red blood cell (RBC) production, increased RBC destruction, or decreased erythropoietin production.1,3 Anemia often goes undiagnosed in many elderly patients due to the ambiguity and non-specific nature of symptoms, such as fatigue, weakness, and dyspnea; however, it can have drastic implications on measures found among the federal nursing home quality indicators, including rates of decline in mobility, cognitive impairment, dementia, and activities of daily living.2,4 Anemia may also increase the risk of falls in elderly patients who reside in the community or in LTCFs.2,4
Such clinical implications may have a direct impact on health care resource utilization (HCRU) and financial outcomes for providers. Elderly patients with anemia have been shown to experience more hospitalizations, longer lengths of stay, and increased severity of chronic comorbidities including diabetes, cardiovascular disease, and chronic kidney disease (CKD), which may in turn lead to higher HCRU and costs. Appropriate management of anemia in the LTC setting is vital to better outcomes for patients and LTCFs alike. Appropriate diagnosis, treatment and follow-up are key areas of opportunity for improving anemia management in this setting.
Management of Anemia in the Elderly
Diagnosis of Anemia
As treatment success is dependent on addressing the underlying etiology of disease, optimal management of anemia requires a thorough and accurate diagnosis.4 The diagnosis of anemia should start with recognition of signs or symptoms warranting further investigation rather than identification through a somewhat randomly obtained complete blood count (CBC).4 When anemia is in the differential diagnosis, a CBC followed by a hemacult can be done to detect gastrointestinal (GI) bleeding, and iron studies can be done to test for iron-deficiency anemia.4
Once a diagnosis has been made, treatment can target the underlying etiology, such as the need for iron replacement. Laboratory results of serum iron can help determine the etiology of anemia; however, patients’ medical history, clinical status, tolerance of treatment, and physician judgment are all key components of individualizing treatment to address the needs of each patient.4 Normal values of pertinent parameters in serum iron tests are presented in Table 1.

Through timely diagnosis and treatment, LTC residents can be cared for within the LTCF, avoiding a costly emergency room and hospital admission—costly in terms of not only financial expense but also the impact on the resident’s quality of life.
Treatment of Anemia
Therapeutic strategies for addressing anemia include treatment of underlying disorder (eg, vitamin B12 deficiency), iron supplementation, erythropoietic agents, and blood transfusions.2 Currently available, FDA-approved pharmacologic options for treatment of anemia are presented in Table 2.

Iron Supplementation. In patients diagnosed with iron-deficiency anemia who are hemodynamically stable, oral iron supplementation is considered first-line therapy, since it is generally safe, inexpensive, and effective in restoring iron balance in the average elderly patient with a chronic GI bleed.5 Oral iron supplements come in two different chemical forms: ferric and ferrous. The ferrous form of iron is more widely utilized as it has higher absorption from the gut.5 The current available oral preparations of iron have varying amounts of elemental iron (Table 3); however, they are all similar in terms of bioavailability.5 The Centers for Disease Control and Prevention (CDC) recommends a daily treatment dose of 150–180 mg of elemental iron, administered in divided doses (2 to 3 times daily).5

Upon initiation of oral iron supplementation, hemoglobin increases are usually seen within 2 to 3 weeks, however, reticulocyte counts may begin to rise within the first week.5 Oral iron therapy may be limited by the extent of GI side effects, including abdominal discomfort, nausea, vomiting, and constipation. Enteric-coated and delayed-release formulation may help reduce GI side effects and increase compliance; however, their absorption is lower compared with that of non-enteric-coated formulations.5 If GI side effects are problematic, an iron product with lower concentration of elemental iron, such as ferrous sulfate or gluconate, or an overall lower dose of elemental iron may be an option; however, it may be inadequate to achieve the desired serum and stored iron levels, especially during initial management.4 Patients with iron deficiency who fail to respond to oral supplementation may be treated with parenteral therapy after careful consideration.4
In patients diagnosed with iron-deficiency anemia with chronic GI bleeding leading to blood loss ≥ 10 ml/day, the use of intravenous (IV) iron therapy can help achieve desired serum iron levels.5 Whereas maximum iron delivery from reticuloendothelial stores is 40-60 mg/day to the bone marrow for RBC production, oral iron supplementation can supply 60–80 mg per day, and IV iron therapy can supply up 160 mg per day.5 Furthermore, whereas the maximum RBC production that can be achieved in patients with serum iron < 70 mcg/100 ml without treatment is about 3.5 times normal, IV therapy can increase production up to ~8 times normal.5 However, this response is usually transient, and excess iron is sequestered and stored in the reticuloendothelial system. Thus, it is imperative for the provider to calculate the total iron deficit in order to decide the appropriate dose of IV therapy (Box 1).5

IV iron preparations available in the United States differ in the concentration of elemental iron (Table 4). These include iron dextran, iron sucrose, sodium ferric gluconate, and ferumoxytol. It is important to note that not all of these preparations are FDA-approved for infusion.

Anaphylactic reactions are a rare but potentially lethal concern with IV iron therapy and typically occur within minutes of administration.6 Providers should closely follow the manufacturer’s recommendation for proper administration, including administration of a test dose and ensuring that personnel are trained to provide emergency treatment in case of a severe reaction.1
Dosage and administration considerations for each IV iron preparation may differ by product (Table 5). As the oldest product in this class, iron dextran carries the highest risk of anaphylactic reactions; however, it has the advantage of total dose infusion (ie, replacing the patient’s total iron requirement in one infusion) and lowest cost.5 Newer products such as iron sucrose and sodium ferric gluconate have a lower incidence of anaphylaxis but are far more expensive and require repeated administrations to replenish iron stores.5 Additional reported adverse events associated with each of the iron preparations include hypotension, arthralgias, myalgias, malaise, abdominal pain, nausea, and vomiting. These non-life-threatening adverse reactions are also more commonly associated with iron dextran and less so with iron sucrose or sodium ferric gluconate.5

The patient’s response to IV iron therapy should be monitored based on the increase in reticulocyte count, which usually occurs 7 to 10 days after initiation of treatment.1 A subnormal response to treatment may occur due to multiple underlying causes, including continued bleeding, insufficient dietary iron intake, hemoglobinopathy, insufficient supplementation, malignancy, or underlying infection.1 The duration of therapy should be determined by factors such as severity and cause of anemia, severity of symptoms, and patient’s hematologic response.6 Treatment usually lasts up to 6 months to fully replenish the body’s stored iron;6 however, exact duration should be determined based on patient-specific factors. Therapy should be discontinued when: (1) the treatment goals are reached; or (2) it is clear that further treatment will not improve the patient’s condition (ie, due to underlying causes of anemia that are not affected by iron supplementation, such as chronic disease).1
Regarding the coverage for iron supplements, it is important to note that Medicare Part D excludes vitamins and minerals from coverage except for prenatal vitamins. For LTC residents who have both Medicare and Medicaid (dual eligibility), coverage for iron supplements is provided under their Medicaid benefit. Appreciation of the coverage for this therapy is important to ensure patients have appropriate access.
Erythropoiesis-Stimulating Agents (ESAs). Deficiency of the hormone erythropoietin is the primary, but not necessarily the sole, cause of anemia associated with CKD. Patients with CKD often have impaired erythropoietin production; however, anemia associated with CKD may stem from other causes, including chronic bleeding and nutritional deficiencies.1 The use of ESAs may be warranted if anemia in older adults is related to one of several causes, including end-stage kidney disease, anemia of chronic disease, or myelodysplastic syndrome (MDS).4
The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guidelines recommend that, prior to initiation of ESA therapy, all correctable causes of anemia (ie, iron deficiency, inflammation) should be addressed.7 Considering that ESA therapy has been found to be associated with increased risk of serious cardiovascular events, stroke, or venous thromboembolism, initiation and maintenance of ESA therapy should compare the potential benefits of fewer blood transfusions and anemia-related symptoms to the risks of ESA therapy.7 Several studies have specifically looked at the safety and efficacy of ESAs in the LTC population and found that there were no significant differences among older patients as compared with younger patients.2
In patients who are appropriate candidates for ESA therapy, goals of treatment should be based on clinical status and whether or not the patient is on dialysis. For patients with CKD not on dialysis, it is recommended to initiate ESA therapy only for hemoglobin (Hb) concentration < 10 g/dL. If the patient meets the specified Hb concentration criteria, decision to initiate and maintain therapy should be based on rate of Hb concentration decline, prior response to iron therapy, risk of needing transfusion, risks of ESA therapy, and presence of symptoms of anemia.7
In patients with CKD who are on dialysis, ESA therapy may be initiated when Hb concentration is between 9 g/dL and 10 g/dL. The goal of treatment in this population should be to prevent the Hb levels from falling below 9 g/dL.7 Guidelines provide a general recommendation for maintenance ESA therapy, stating that ESAs should not be used to maintain Hb concentration above 11.5 g/dL. Exceptions may be made on a case-by-case basis for patients who require higher levels of Hb concentrations to achieve a meaningful improvement in quality of life and fully understand the risks associated with ESA therapy. Under no circumstances should ESAs be used to intentionally increase the Hb concentration above 13 g/dL due to the drastic increase in thromboembolic complications.1,7
ESAs that are FDA-approved and available in the United States are listed in Table 6. Dosing for ESA therapy should be based on patients’ current Hb level, body weight and clinical status.7 The available ESAs are largely similar in their indications and dosing requirements; however, minor differences may exist with regards to exact indications, dose, frequency, and clinical considerations (Table 6).
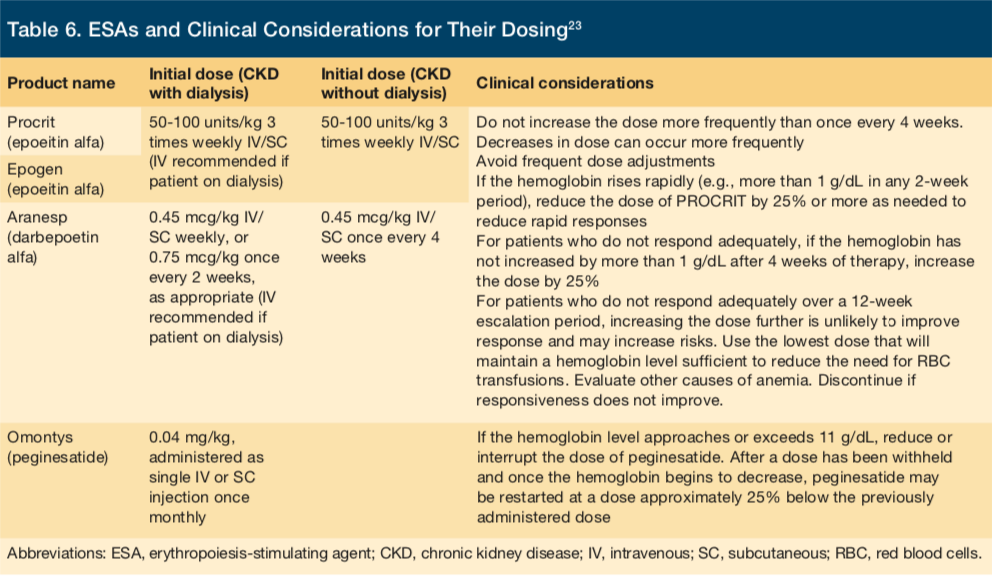
ESAs may be administered subcutaneously or intravenously, depending on CKD stage, treatment setting, efficacy, safety, convenience, and the ESA agent used. Dose adjustments for ESA therapy should be based on similar factors as those considered during initiation of therapy, including current Hb level, rate of change in Hb levels, current ESA dose, and clinical circumstances.8 When a downward adjustment is needed, decreasing the ESA dose is preferred over withholding therapy altogether. In the case that a patient experiences an ESA-related adverse event or has an acute or progressive illness that may cause ESA hyporesponsiveness—defined as no increase in Hb level from baseline after the first month of ESA treatment with appropriate weight-based dosing)—the dose and continuation of ESA therapy should be re-evaluated.9
Guidelines recommend that, during the initiation phase of ESA therapy, Hb levels should be measured at least monthly;1 however, the FDA advises that after initiation of ESA therapy, Hb levels should be checked twice per week for 2-6 weeks to ensure that levels have stabilized.1 In the case that the patient’s Hb level increases by more than 1 g/dL in a 2-week period, the ESA dose should be decreased.1 In the maintenance phase, Hb levels for patients with CKD who are not on dialysis should be monitored at least every 3 months, whereas the recommended interval is monthly for patients with CKD who are on dialysis.1
As with iron supplements, coverage for ESAs is complex. Except for dialysis patients, coverage for ESAs for most LTC residents will occur under Medicare Part D. In the case of those LTC residents receiving dialysis, ESAs are the responsibility of the dialysis center, as it is included in their capitated payment for dialysis and related care. Knowing this—particularly for residents receiving sub-acute care—will allow the nursing home to avoid paying for a treatment that is not their responsibility. In today’s environment of increasing financial pressures, this knowledge is increasingly critical.
Blood Transfusions. Transfusion of blood product has historically been done at hospitals and transfusion centers. However, there is strong demand by the nursing home industry to start in-house transfusion for hemodynamically stable patients. As optimum transfusion practice is under intense review by regulatory agencies,10 it is important for physicians practicing in LTCFs to become familiar with indications, complications, and hazards of transfusions. Fatalities associated with blood product transfusions are rare;11 however, they do happen. Testing for infectious diseases (including syphilis, hepatitis B virus, hepatitis C virus, human immunodeficiency virus strains 1 and 2, human T-cell lymphotropic virus, West Nile virus, and Chagas disease) has created much safer practice.12
Nursing homes are encouraged to offer blood and platelet transfusions to their patients.13 However, the entire process—from obtaining consent, type-cross-matching, and actual transfusion—should not be more than 24 hours and must be done by nursing staff who have had adequate training in this field.14 Medical and nursing staff should be educated on transfusion reactions including hemolytic, non-hemolytic, allergic, anaphylactic, transfusion-related acute lung injury, delayed immunologic reactions, infections, circulatory overload, and hypothermia. Also, indications for irradiated blood and/or warmer blood need to be discussed.15
Medical directors, administrators, directors of nursing, and blood bank representatives all need to be actively involved and should participate in the transfusion committee. This committee is responsible for reviewing all transfusions for indications, adverse events, and clinical errors. Policies and procedures need to be reviewed and periodically updated by the transfusion committee.16
Future of Anemia Management in LTC
As LTC providers increasingly become responsible for delivery of outcomes in an efficient and effective manner, the management of disease states like anemia will require a system-wide approach. This approach needs to have a focus on individual patient quality while being cost effective. In order to accomplish this, LTC providers must develop a system for their facility that starts with appropriateness of diagnosis of anemia followed quickly by identification and treatment of the underlying cause. Some of the most common conditions will involve either a blood loss or deficiency. The treatments may involve replacement with either iron or blood or stimulation of erythropoietin production. No matter the treatment course, having a well-defined system to manage anemia in LTCFs will prevent unnecessary transfers to the emergency room, providing a better quality of life for residents and at a lower cost to the health system.
1. American Medical Directors Association (AMDA). Anemia in the long-term care setting. Columbia (MD): American Medical Directors Association (AMDA); 2007.
2. Tangalos E, Zarowitz B, Gunter D, McClellan WI. New directions: management of anemia in long-term care. Anemia in Long Term Care. http://medicine.emory.edu/ger/edu_resources/module/module12_files/management_of_anemia_monograph_final.pdf. Accessed August 31, 2016.
3. World Health Organization. The Global Prevalence of Anaemia in 2011. 2015. http://apps.who.int/iris/bitstream/10665/177094/1/9789241564960_eng.pdf.
4. Goodnough LT, Schrier SL. Evaluation and management of anemia in the elderly. Am J Hematol. 2014;89:88–96.
5. Johnson-Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Therap Adv Gastroenterol. 2011;4(3):177-184.
6. Schrier S, Auerbach M. Treatment of iron deficiency anemia in adults. Up to Date. http://www.uptodate.com/contents/treatment-of-iron-deficiency-anemia-in-adults. Updated August 17, 2016. Accessed August 31, 2016.
7. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Inter., Suppl. 2013;3:1-150.
8. FDA Drug Safety Communication: Modified dosing recommendations to improve the safe use of Erythropoiesis-Stimulating Agents (ESAs) in chronic kidney disease. Drug Safety and Availability. http://www.fda.gov/drugs/drugsafety/ucm259639.htm. Published April 1, 2016. Accessed August 26, 2016.
9. Johnson DW, Pollock CA, Macdougall IC. Erythropoiesis-stimulating agent hyporesponsiveness. Nephrology. 2007;12(4):321-330.
10. AABB, American Red Cross, America’s Blood Centers, Armed Services Blood Program. Circular of Information for the Use of Human Blood and Blood Components. aabb.org/resources/bct/Pages/aabb_coi.aspx. Published April 2013. Accessed August 31, 2016.
11. Kleinman S, Chan P, Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17:120-162.
12. Zou S, Stramer SL, Dodd RY. Donor testing and risk: Current prevalence, incidence and residual risk of transfusion- transmissible agents in U.S. allogeneic donations. Transfus Med Rev. 2012;26:119-128.
13. Zou S, Dorsey KA, Notari EP, et al. Prevalence, incidence, and residual risk of human immunodeficiency virus and hepatitis C virus infections among United States blood donors since introduction of nucleic acid testing. Transfusion. 2010;50:1495-1504.
14. Eder AF, Chambers LA. Noninfectious complications of blood transfusion. Arch Pathol Lab Med. 2007;131:708-718.
15. Roback JD,Grossman BJ Harris T, Hillyer CD, eds. Technical Manual. 17th ed. Bethesda, MD: AABB Press, 2011.
16. Carson TH, ed. Standards for Blood Banks and Transfusion Services, 28th edn. Bethesda, MD: AABB Press, 2012.
17. Gerstan T. Serum iron test. MedlinePlus Medical Encyclopedia. https://medlineplus.gov/ency/article/003488.htm. Published February 24, 2014. Accessed August 26, 2016.
18. Amgen. Aranesp Label. http://www.accessdata.fda.gov/drugsatfda_docs/label/2002/darbamg071902lb.pdf.
19. Amgen. Epoetin alpha Prescribing Information. Epogen. http://pi.amgen.com/united_states/epogen/epogen_pi_hcp_english.pdf.
20. Beck RK. Drug Reference for EMS Providers. Albany: Delmar Thomson Learning; 2002.
21. VENOFER Prescribing Information. VENOFER- iron sucrose injection, solution. http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/pediatricadvisorycommittee/ucm437786.pdf.
22. Short MW, Domagalski JE. Iron deficiency anemia: evaluation and management. Am Fam Physician. 2013;87(2):98-104.
23. Gahart B, Nazareno A. 2016 Intravenous Medications: A Handbook for Nurses and Health Professionals. Elsevier Health Sciences; 2016.









