LTC Bulletin Board - July 2011
FDA Approves New Treatment for Preventing Blood Clots After Joint Surgery
On July 1, 2011, the FDA approved Xarelto (rivaroxaban), an oral, once-daily anticoagulant, to reduce the risk of blood clots, deep vein thrombosis (DVT), and pulmonary embolism (PE) following knee or hip replacement surgery. More than 800,000 Americans undergo knee or hip replacement surgeries each year, which require an average hospital stay of 3 to 5 days. Following hospital discharge, the American College of Chest Physicians recommends continuing anticoagulant therapy for at least 10 days for knee replacements and up to 35 days for hip replacements to help reduce the risk of DVT and PE, which are the leading causes of rehospitalization in patients undergoing these procedures.
“Shorter hospital stays following hip and knee replacement surgeries have made the prevention of venous blood clots an outpatient issue, and Xarelto provides a safe and effective oral treatment option that can be easily transitioned from use in hospital to home,” said Paul Chang, MD, vice president, Medical Affairs, Internal Medicine, Janssen Pharmaceuticals, Inc., in a press release.
The FDA approved Xarelto for use at a 10-mg dose following a clinical study of more than 6000 patients undergoing knee or hip replacement surgeries. The studies were designed to identify occurrences of venous thromboembolic events (VTE), such as DVT and PE, or death in patients treated with Xarelto, compared with those treated with Lovenox (enoxaparin), another FDA-approved drug that prevents clotting.
The study showed, overall, that fewer patients being treated with Xarelto had VTE compared with those treated with Lovenox. Data from the phase 3 clinical development program showed significantly greater efficacy of Xarelto, both in head-to-head comparison with Lovenox and when comparing 5-week extended-duration Xarelto with 2-week short-term Lovenox, followed by placebo.
The most commonly reported side effect of using Xarelto was episodic bleeding. Xarelto is also being evaluated as a prophylactic and treatment for other disorders in which blood clotting plays a major role, such as acute coronary syndrome.
FDA Approves New Treatment for Pulmonary Disease
On July 1, 2011, the FDA approved Arcapta Neohaler (indacaterol inhalation powder), the first once-daily, long-acting beta2 adrenergic agonist (LABA) approved to treat airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema. Arcapta Neohaler helps muscles around the airways of the lungs stay relaxed to prevent COPD symptoms, such as wheezing and breathlessness. It is not intended to treat asthma or sudden, severe symptoms of COPD.
“The approval of new long-term drugs for COPD that relieve breathing difficulty by opening airways provides another treatment option for millions of people,” said Curtis Rosebraugh, MD, MPH, director, Office of Drug Evaluation II, Center for Drug Evaluation and Research, in an FDA statement.
The FDA’s approval of Arcapta Neohaler was based on the safety and efficacy observed with the drug in six confirmatory clinical trials that included a cumulative 5474 COPD patients ≥40 years of age. Those treated had a history of smoking at least one pack a day for 10 years and exhibited moderate-to-severe decreases in lung function.
The most common side effects reported by patients were runny nose, cough, sore throat, headache, and nausea. As with all LABAs, Arcapta Neohaler may increase the risk of asthma-related death and should not be used in patients with asthma, unless used with a long-term asthma control medication.
Novartis is anticipating an early 2012 launch of Arcapta Neohaler in the United States. The drug is already approved as a COPD treatment in more than 60 countries and is currently available in approximately 30. For more information, visit the Arcapta Website (https://bit.ly/Arcapta).
FDA Modifies Dosing for Treating Chronic Kidney Disease
On June 24, 2011, the FDA announced more conservative dosing guidelines for erythropoiesis-stimulating agents (ESAs) when used to treat anemia in patients with chronic kidney disease (CKD), eliminating the concept of a target hemoglobin range of 10 g/dL to 12 g/dL. In addition, the prescribing information and boxed warning will now differentiate between dosing strategies for patients receiving and not receiving dialysis.
Modified language to the prescribing information and boxed warning is being added to reflect the results from clinical trials, including TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy), which showed that using ESAs to target hemoglobin levels of ≥11 g/dL increased the risk of serious and adverse cardiovascular events and provided no additional benefit to patients.
In the new guidelines, the FDA advises physicians to start ESA treatment in patients on dialysis when their hemoglobin level is <10 g/dL, and to reduce or interrupt the dose once their hemoglobin level approaches or exceeds 11 g/dL. For those not on dialysis, the FDA recommends starting ESA therapy when their hemoglobin level is <10 g/dL and the risk for red blood cell transfusion is high, and reducing or interrupting the dose when their hemoglobin level is >10 g/dL.
“The goal is to individualize therapy and use the lowest ESA dose possible to reduce the need for red blood cell transfusions,” said John Jenkins, MD, director, Office of New Drugs, Center for Drug Evaluation and Research, in an FDA statement.
The FDA will continue to evaluate the safety of ESAs and is requiring their manufacturer, Amgen Inc., to conduct additional trials. It is also approving modifications to the existing Risk Evaluation and Mitigation Strategy for these agents.
FDA Approves First Generic Versions of Levofloxacin
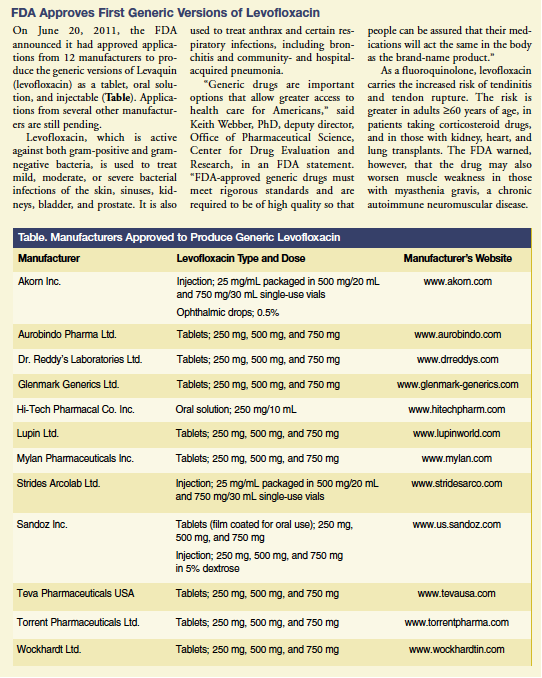
On June 20, 2011, the FDA announced it had approved applications from 12 manufacturers to produce the generic versions of Levaquin (levofloxacin) as a tablet, oral solution, and injectable (Table). Applications from several other manufacturers are still pending.
Levofloxacin, which is active against both gram-positive and gram-negative bacteria, is used to treat mild, moderate, or severe bacterial infections of the skin, sinuses, kidneys, bladder, and prostate. It is also used to treat anthrax and certain respiratory infections, including bronchitis and community- and hospital-acquired pneumonia.
“Generic drugs are important options that allow greater access to health care for Americans,” said Keith Webber, PhD, deputy director, Office of Pharmaceutical Science, Center for Drug Evaluation and Research, in an FDA statement. “FDA-approved generic drugs must meet rigorous standards and are required to be of high quality so that people can be assured that their medications will act the same in the body as the brand-name product.”
As a fluoroquinolone, levofloxacin carries the increased risk of tendinitis and tendon rupture. The risk is greater in adults ≥60 years of age, in patients taking corticosteroid drugs, and in those with kidney, heart, and lung transplants. The FDA warned, however, that the drug may also worsen muscle weakness in those with myasthenia gravis, a chronic autoimmune neuromuscular disease.
Policy News
New Act Yields Big Savings on Prescription Drugs
Approximately half a million people are receiving cheaper prescription drugs, thanks to the Affordable Care Act. In June 2011, the Centers for Medicare & Medicaid Services (CMS) announced that about 478,000 people with Medicare Part D who reached the coverage gap known as the “donut hole” received an automatic 50% discount on their covered brand-name prescription drugs. The beneficiaries received the discount in the first 5 months of 2011, resulting in total savings of $260 million, or an average savings of $545 per beneficiary. Most of the discounts are helping Americans with serious medical conditions. About $36 million was spent on cancer drugs, $21 million on drugs to control hypertension and cholesterol levels, and about $20 million on diabetes medications.
“Without the Affordable Care Act, many seniors and people with disabilities would pay twice as much for their prescription drugs in the donut hole,” said CMS Administrator Donald M. Berwick, MD, in a press release. In May 2011, the number of beneficiaries who received the discount rose by ≥76% and the dollar amount of savings rose by ≥56%. CMS expects that as many as 4 million more beneficiaries will fall into the coverage gap later this year and benefit from these discounts. An analysis from November 2010 by the U.S. Department of Health and Human Services estimated that Medicare improvements due to the Act would provide average per-person savings of more than $3500 over the next 10 years. Those with higher drug costs could see savings as high as $12,000.
For more information about the drug discount and various provisions of the Affordable Care Act, visit www.healthcare.gov.
Alzheimer’s News
Patent Dispute Detracts from Alzheimer’s Research
Transgenic mouse models are at the center of a lawsuit that is threatening a community of Alzheimer’s disease researchers. According to a report by Nature News, the Alzheimer’s Institute of America (AIA) is involved in litigation against Jackson Laboratory, a Maine-based source of laboratory mice funded by the National Institutes of Health (NIH).
In 2010, AIA filed a lawsuit claiming Jackson infringed upon its Swedish mutation patent when it sold and distributed 22 strains of mice with the mutation to researchers. The AIA holds a patent on a human DNA sequence used in mice to trigger early onset of Alzheimer’s, and the NIH requires scientists to share transgenic mouse strains developed using NIH funds. The AIA claims its mouse model patents may not be used for profit; they may only be used for academic purposes.
“Jackson Laboratory is not giving away the mice for academic research. On the contrary, these mice are being sold, and Jackson Laboratory is making quite a lot of money in the process,” the AIA wrote in a statement. Jackson has denied selling the mice to companies.
Nature reported that the AIA would drop the lawsuit if Jackson turns over names of the scientists who received the mice, but doing so could put the researchers at risk for litigation. Jackson officials say that Jackson only allows academics, and not companies, to use the models. But litigation to disprove AIA’s claims could cost Jackson years and a wealth of resources. The laboratory has asked NIH to assume the cost of its defense.
“We haven’t been able to settle this case because we’re trying to do the right thing by trying to support the NIH policy and protect researchers out there in the community,” said David Einhorn, Jackson house counsel, in Nature.
Read the entire report online at www.nature.com/news/2011/110405/full/472020a.html.
New Alzheimer’s Research Suggests Presymptomatic Detection Possible
New criteria and guidelines for diagnosing Alzheimer’s disease have been published for the first time in nearly 30 years. Led by the Alzheimer’s Association and the National Institute on Aging of the National Institutes of Health, a team of researchers has published several articles that expand the definition of Alzheimer’s to include a preclinical stage. The new guidelines also refine the existing guidelines for diagnosing mild cognitive impairment (MCI) and establish a framework for monitoring certain biomarkers to diagnose Alzheimer’s at every stage.
Reflecting an emerging consensus, the authors propose that Alzheimer’s begins with a long, asymptomatic period, during which measurable changes in the brain can be detected years and even decades before symptoms of memory and thinking lapses are noticeable. Researchers are hopeful that biomarkers detected with brain imaging studies and cerebrospinal fluid assays will move the field toward earlier diagnosis and more precise, effective treatment. However, the use of biomarkers in Alzheimer’s dementia and MCI was proposed as a research agenda only and is not intended for application in a clinical setting until additional research has been conducted. The guidelines do not yet specify which biomarkers should be considered as signature indications of preclinical Alzheimer’s.
“Currently, Alzheimer’s therapies are in development that may be able to slow or stop the progression of the disease. By improving early detection and risk evaluation, we will better be able to test potential therapies and eventually prescribe them for people at increased risk,” said William Thies, PhD, chief medical and scientific officer, Alzheimer’s Association, in a press statement.
The articles also formalize the distinction that everyone who develops Alzheimer’s experiences a preceding MCI stage, but not everyone with MCI will receive an Alzheimer’s diagnosis because MCI may occur for other reasons. The new guidelines define the condition of “MCI due to Alzheimer’s disease” and outline four levels of certainty for diagnosis. One of the workgroups was charged with examining postmortem, pathological criteria for Alzheimer’s. The results of its research are expected to appear later this year.
The new guidelines can be downloaded at www.alz.org/research/diagnostic_criteria.