Can Probiotics Prevent Antibiotic- or Clostridium difficile-Associated Diarrhea in Long-Term Care Residents?
In long-term care (LTC) facilities, the pathogen Clostridium difficile and widespread use of broad-spectrum antibiotics are among the most common causes of diarrhea, which is defined as “watery or unformed stools that occur more than three times daily for at least 2 days.” These antibiotics often disrupt intestinal microflora, which generally serve as a protective barrier against colonization by intestinal pathogens.1 The absence of these microflora leaves patients susceptible to C. difficile and other opportunistic pathogens associated with intestinal infections. These infections and the antibiotics required to treat them can lead to a diagnosis of antibiotic-associated diarrhea (AAD).2,3
Broad-spectrum antibiotics, such as amoxicillin, second- and third-generation cephalosporins, and clindamycin, pose the greatest risk for development of AAD.2 A diagnosis of AAD is typically applied to patients with diarrheal illness who have taken antibiotics within the past 2 months and have no other identifiable cause of diarrhea. Between 8% and 33% of LTC residents treated with antibiotics acquire C. difficile infection.4 Although not the only cause of AAD, C. difficile is the most common cause of acute diarrhea in nursing home residents and coincides with antibiotic use in 80% of cases.4
Elderly individuals, especially those residing in LTC facilities, are at especially high risk for super infections of C. difficile because of their advanced age, frequent use of antibiotics, and multiple comorbidities. A study of LTC residents at one Canadian nursing home found that 2.1% to 8% of patients developed C. difficile-associated diarrhea (CDAD) annually.5 In 2006, a study evaluating the rate of CDAD among residents at a US nursing home reported 0 to 2.62 cases per 1000 resident days and noted that 21.7% of residents experienced CDAD recurrence.4 CDAD is associated with a 1% to 2.5% mortality rate, underscoring the importance of prompt diagnosis and treatment.6 CDAD is diagnosed when a stool culture is positive for toxins A or B, a stool cytotoxicity assay is positive for toxin B (Figure), or an endoscopy shows colonic pseudomembranes. Current recommendations for treating CDAD call for oral metronidazole for mild-to-moderate cases and oral vancomycin for severe cases.
The most effective way to prevent CDAD and AAD is through judicious use of antibiotics, especially broad-spectrum antibiotics such as quinolones, but these agents often cannot be avoided. Probiotics have been assessed as prophylaxes for CDAD and AAD, but study findings have been inconsistent. A review of the most relevant literature was conducted to determine whether administering probiotics when administering antibiotic therapy might benefit geriatric patients.
Method
CINAHL, MEDLINE, PubMed, Cochrane Systemic Review, and Cochrane Central Register of Controlled Trials were searched for the following terms: probiotics, diarrhea, and antibiotic C. difficile, which yielded 302 studies. For a clinical trial to satisfy inclusion criteria, enrollment had to include adults, reflect evidence-based practice, follow practice protocols, and be published in the English-language literature. Studies were excluded if they used probiotics to treat AAD and CDAD, enrolled only children, or consisted of a literature review. The 51 studies meeting these inclusion criteria were screened to determine if their objective was to evaluate whether administering a probiotic during antibiotic treatment would decrease the incidences of AAD and CDAD in an adult population. Of these, 6 randomized controlled trials, 4 meta-analyses, and 1 systematic review of randomized controlled trials met all predefined inclusion criteria, cumulatively providing data for >5000 treated patients.
Review of the Literature
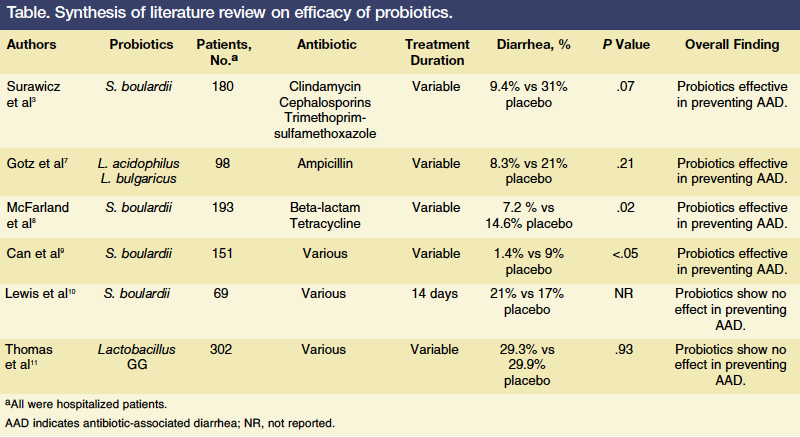
This literature review is divided into three sections. The first examines randomized controlled trials that support the use of probiotics (n = 4), the second examines randomized controlled trials that did not support the use of probiotics (n = 2), and the third outlines findings from the 4 meta-analyses and the 1 systematic review on the use of probiotics. The 6 randomized controlled trials are summarized in the Table.
Studies Supporting Probiotic Use
In 1979, Gotz and colleagues7 reported data from a trial that included 98 hospitalized patients (age, 18-88 years) who were randomized to receive placebo or a preparation combining Lactobacillus acidophilus and L. bulgaricus during the first 5 days of treatment with ampicillin. Of the 79 patients for whom complete data were available, 36 received the probiotic preparation four times daily and 43 received placebo four times daily. After excluding the 50% of patients with diarrhea identified as unrelated to ampicillin, AAD was found to have occurred in 14% of patients taking placebo and 0% of patients given probiotics (P = .03). Based on these findings, the investigators concluded that prophylactic administration of Lactobacillus preparations might be effective at preventing ampicillin-induced diarrhea.
In 1989, Surawicz and colleagues3 published findings from a double-blind randomized trial of 180 hospitalized adults taking antibiotics (eg, clindamycin, cephalosporins, trimethoprim-sulfamethoxazole) over a 23-month period who were randomly assigned to receive placebo or Saccharomyces boulardii, a probiotic. The duration of antibiotic use varied between participants. The investigators found that 22% of patients who received placebo developed AAD compared with only 9.5% of patients given the probiotic (P = .038). When examining the effect of S. boulardii on patients who tested positive for C. difficile infection (n = 48), 31% (5 of 16) in the placebo group developed diarrhea compared with 9.4% (3 of 32) in the probiotic group, a finding that did not reach statistical significance. Based on their overall findings, Surawicz and colleagues concluded that S. boulardii reduces the incidence of AAD in hospitalized patients receiving antibiotics.
The results of another randomized placebo-controlled trial assessing the efficacy of S. boulardii as a prophylactic for AAD were reported by McFarland and colleagues8 in 1995. In this trial, 193 patients were treated with at least one beta-lactam antibiotic and received placebo (n = 96) or 1 g of S. boulardii (n = 97) daily. Patients started on the probiotic (or placebo) within 72 hours of initiating antibiotic therapy, took it daily while receiving the antibiotic, and discontinued it 3 days after completing antibiotic therapy. Patients were followed for 7 weeks after antibiotic therapy ended. In the group of patients taking S. boulardii, 7.2% developed AAD compared with 14.6% of patients given placebo (P = .02). The efficacy of S. boulardii in preventing AAD was calculated at 51%. McFarland and colleagues concluded that the prophylactic use of S. boulardii in conjunction with a beta-lactam antibiotic significantly reduces AAD without the risk of serious adverse reactions.
The most recent randomized, placebo-controlled trial to meet the inclusion criteria was published in 2006 by Turkish researchers and included complete data for 151 hospitalized patients aged 25 to 50 years who were not pregnant or lactating and had no chronic illnesses.9 Patients were randomized to receive S. boulardii or placebo along with their antibiotic therapy. The study found that 7 (9%) of the 78 patients receiving the placebo developed AAD compared with 1 (1.4%) of the 73 patients taking the probiotic (P <.05). Stool samples were collected for all patients who developed AAD. C. difficile infection was identified in 2 of 7 stool samples collected from the placebo group. No stool samples from the probiotic group tested positive for C. difficile. The results implied that prophylactic use of S. boulardii prevented AAD in hospitalized patients without causing any serious side effects. Based on their findings, the investigators concluded that probiotics are more effective than placebo at preventing AAD and CDAD.
Studies Not Supporting Probiotic Use
In 1998, Lewis and colleagues10 reported data from a trial that determined S. boulardii was ineffective at preventing AAD. Their randomized study involved 72 consecutively hospitalized patients aged ≥65 years who were randomly assigned to receive S. boulardii 113 mg twice daily or placebo for the duration of their antibiotic regimen. Patients’ bowel habits were closely monitored, and stool samples were tested every fourth day for C. difficile toxins. A total of 69 patients completed the study. Similar numbers of patients in each group experienced watery stools or tested positive for C. difficile toxin. Lewis and colleagues said their study found no evidence that S. boulardii prevents AAD or CDAD, and they suggested probiotics be properly evaluated before they are widely prescribed in medical practice.
In 2001, Thomas and colleagues11 reported the results of their randomized, double-blind, placebo-controlled trial, which included 302 hospitalized patients receiving antibiotics with placebo (n = 134) or Lactobacillus GG (n = 133) for 14 days. Patients were instructed to record their bowel movements for 21 days. Diarrhea was reported by 39 patients (29.3%) in the Lactobacillus GG group and 40 patients (29.9%) in the placebo group. While Lactobacillus GG did not reduce the incidence of AAD, only a few patients had stool cultures or additional laboratory tests for diarrhea and there were too few positive tests for C. difficile infection to determine whether Lactobacillus GG might have prevented colonization of this pathogen.
Meta-analyses and Systematic Review Findings
Four meta-analyses and 1 systematic review were also reviewed to evaluate the effects of probiotics in preventing AAD or CDAD. The first meta-analysis was published in 2002 and reviewed 9 randomized placebo-controlled trials that cumulatively included >1300 patients; 2 of the 9 studies enrolled children.12 Various probiotics were evaluated, with 4 studies using S. boulardii, 4 using lactobacilli, and 1 using a strain of Enterococcus that produces lactic acid. In the meta-analysis, the combined odds ratio (OR) favored active treatment over placebo, with an OR of 0.37 (95% confidence interval [CI], 0.26-0.53; P <.001). The authors tested for publication bias using a funnel plot of ORs against sample size, the Begg and Mazumdar adjusted rank correlation test, and the Egger et al regression asymmetry test. Based on the results of these three tests, the investigators concluded that publication bias was not present in their meta-analysis. They also noted that while S. boulardii and lactobacilli may prevent AAD, additional studies would be needed to determine whether routine use of these agents is economically feasible.
The second meta-analysis examined the results of 7 randomized placebo-controlled trials that included a cumulative 800 patients and evaluated the effectiveness of Lactobacillus spp. and Saccharomyces spp. versus placebo at preventing AAD.13 Publication bias was assessed using a funnel scatterplot that plotted ORs of the studies against their sample sizes. The investigators found that both Lactobacillus spp. and Saccharomyces spp. were more effective than placebo at preventing AAD. While the findings suggest patients receiving antibiotics benefited from probiotic use, the investigators conceded that the overall results of their meta-analysis were questionable because the individual studies had diverse populations, including pediatric participants and individuals from developing countries, and lacked standardized protocols for administering probiotics. The investigators recommend further evaluation of probiotic use to prevent AAD, including cost-benefit analyses.
The third meta-analysis evaluated data from 34 masked, randomized placebo-controlled trials involving >2000 patients between the ages of 6 months and 80 years to determine the impact of probiotics on acute diarrhea.14 Of these trials, 33 were conducted in a healthcare setting in a developed country. The investigators found that probiotics reduced the rate of AAD by 52% (95% CI, 35%-65%). While there was considerable variation between included studies in the probiotic formulation used and its dose and frequency, the protective effect did not vary significantly among the strains assessed, with S. boulardii, L. rhamnosus GG, L. acidophilus, and L. bulgaricus showing similar efficacy when used alone or with at least one other strain. Publication bias was evaluated and found not to have a significant impact on the findings.14
The fourth meta-analysis included 31 randomized controlled trials that studied the use of probiotics in the prevention of AAD and the treatment of CDAD.15 Pediatric, adult, and elderly participants treated at hospitals or outpatient sites were included in these trials. In total, 25 of the studies, which included >2800 subjects cumulatively, investigated using probiotics as a prophylactic against AAD. The authors found that 13 (52%) of these trials demonstrated a significant reduction in the incidence of AAD with probiotic use versus placebo (relative risk, 0.43; 95% CI, 0.31-0.58; P <.001); higher probiotic doses corresponded with greater efficacy. Sixteen of the trials enrolled adults, and 7 (44%) of these showed a reduction in AAD with probiotic use.15 The 31 studies in this meta-analysis used a variety of probiotics, including L. rhamnosus GG and S. boulardii, as well as probiotic mixtures. Although several showed promise as effective therapies for reducing the incidence of AAD, only S. boulardii was found to be an effective treatment for CDAD.
Dendukuri and colleagues16 conducted a systematic review of 8 randomized controlled trials to identify studies in which probiotic therapy to prevent or treat CDAD in hospitalized patients was the primary or secondary objective. The investigators found no evidence that probiotics provided effective prophylaxis; however, the study’s findings were limited by the lack of CDAD cases. The authors also noted that the heterogeneity in the probiotic choice, dose, and criteria for diagnosing CDAD made it difficult to synthesize data from these studies.
Study Strength and Weaknesses
The primary strength of the studies discussed is that they are all randomized controlled trials or assessed randomized controlled trials, allowing for identification of those interventions that contributed to the dependent variables. Overall, there are also several weaknesses, including variability in the study locations, with some trials conducted in developing countries; highly varied study populations, with some that included pediatric patients; use of multiple probiotics or probiotic combinations; and lack of standard probiotic use (ie, strain, dosing, duration of therapy), which would be essential to determine the appropriate use of probiotics in clinical practice. Another major weakness is considerable variation between studies in the duration of follow-up. Many trials followed patients for only a short time after they completed antibiotic therapy, yet it can take as long as 2 months after antibiotic use for AAD or CDAD to develop.
While the majority of studies included in this review demonstrated some benefit to using probiotics in patients receiving antibiotics, it is unclear whether their results can be generalized to the LTC population, as none of the studies were conducted in the LTC setting. However, because AAD and CDAD infections are common among nursing home residents and constitute a serious health problem that results in considerable morbidity and mortality, preventing and effectively treating AAD and CDAD in this patient population is imperative.
Suggested Cost-Effectiveness of Probiotic Therapy
AAD and CDAD can be costly to treat. Common regimens include metronidazole 500 mg orally every 6 hours for 14 days (36-40 pills), which costs approximately $256, or intravenous metronidazole 500 mg every 8 hours for 4 days, which costs approximately $678.17 Another therapeutic option is oral vancomycin 500 mg, administered every 6 hours for 14 days, which significantly increases drug costs to approximately $1724.17 In contrast, probiotics generally cost <$1 dollar per pill and have not been found to produce side effects.
A 2007 study by Hickson and associates18 estimated the average cost of probiotic use at $20 per patient, assuming a 10-day course of antibiotics with 17 days of probiotic therapy. Based on the number of patients needed to treat to prevent one case of AAD or CDAD, they calculated it would cost $100 to prevent a single case of AAD and $120 to prevent a case of CDAD (excluding administration costs). The researchers estimated each case of CDAD prevented in the United States would save an average of $3669, assuming longer hospitalization and treatment with vancomycin. The lack of information on the cost-effectiveness of using probiotics to prevent AAD and CDAD indicates the need for more recent studies to assess whether widespread prophylaxis with probitiocs translates to savings of money and time, particularly in the LTC setting.
Conclusion
Studies on the effectiveness of probiotics in preventing AAD or CDAD have been conducted for >20 years, but the results have not been definitive. Existing clinical trials provide some support for the concomitant use of probiotics with antibiotic administration to reduce the incidence of AAD and CDAD. These studies, however, have not included sufficient numbers of older adults, especially those in LTC settings, to allow direct assessment of the use of probiotics in geriatric nursing home patients. Because AAD and CDAD infections are common among nursing home residents and constitute a serious health problem that results in considerable morbidity and mortality, preventing AAD and CDAD in this patient population is imperative.
Additional studies specifically investigating the use of probiotics in the LTC population and their cost-effectiveness are needed so that healthcare providers caring for LTC residents at risk for AAD or CDAD can make evidence-based treatment decisions. Although these studies have yet to be conducted and findings have yet to be published, the limited evidence available in support of the safety and efficacy of probiotics and their potential to reduce costs suggests administering probiotics to prevent AAD and CDAD in elderly patients receiving antibiotics should be considered.
The author reports no relevant financial relationships. Dr. Edwards-Marshall is an adult nurse practitioner, Department of Geriatrics, Osler Medical, Melbourne, FL.
References
1. Marteau PR, de Vrese M, Cellier CJ, Schrezenmeir J. Protection for gastrointestinal diseases with the use of probiotics. Am J Clin Nutr. 2001;73(2 suppl):430S-436S.
2. Bergogne-Bérézin E. Treatment and prevention of antibiotic associated diarrhea. Int J Antimicrob Agents. 2000;16(4):521-526.
3. Surawicz CM, Elmer GW, Speelman P, et al. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective study. Gastroenterology. 1989;96(4):981-988.
4. Laffan AM, Bellantoni MF, Greenough WB 3rd, Zenilman JM. Burden of Clostridium difficile-associated diarrhea in a long-term care facility. J Am Geriatr Soc. 2006;54(7):1068-1073.
5. Simor AE, Yake SL, Tsimidis K. Infection due to Clostridium difficile among elderly residents of a long-term-care facility. Clin Infect Dis. 1993;17(4):672-678.
6. Schroeder MS. Clostridium difficile-associated diarrhea. Am Fam Physician. 2005;71(5):921-928.
7. Gotz V, Romankiewicz JA, Moss J, Murray HW. Prophylaxis against ampicillin-associated diarrhea with a lactobacillus preparation. Am J Hosp Pharm. 1979;36(6):754-757.
8. McFarland LV, Surawicz CM, Greenberg RN, et al. Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol. 1995;90(3):439-448.
9. Can M, Beşirbellioglu BA, Avci IY, et al. Prophylactic Saccharomyces boulardii in the prevention of antibiotic-associated diarrhea: a prospective study. Med Sci Monit. 2006;12(4):PI19-PI22.
10. Lewis SJ, Potts LF, Barry RE. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J Infect. 1998;36(2):171-174.
11. Thomas MR, Litin SC, Osmon DR, et al. Lack of effect of Lactobacillus GG on antibiotic-associated diarrhea: a randomized, placebo-controlled trial. Mayo Clinic Proc. 2001;76(9):883-889.
12. D’Souza AL, Rajkumar C, Cooke J, Bulpitt CJ. Probiotics in prevention of antibiotic associated diarrhoea: meta-analysis. BMJ. 2002;324(7350):1361-1364.
13. Cremonini F, Di Caro S, Nista EC, et al. Meta-analysis: the effect of probiotic administration on antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2002;16(8):1461-1467.
14. Sazawal S, Hiremath G, Dhingra U, et al. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006;6(6):374-382.
15. McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101(4):812-822.
16. Dendukuri N, Costa V, McGregor M, Brophy J. Probiotic therapy for the prevention and treatment of Clostridium difficile-associated diarrhea: a systematic review [published correction appears in CMAJ. 2005;173(4):345]. CMAJ. 2005;173(2):167-170.
17. Schroeder MS. Clostridium difficile–associated diarrhea. Am Fam Physician. 2005;71(5):921-928.
18. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335(7610):80-83.










