Beriberi in a Malnourished Woman After an Extended Period of Nausea and Vomiting
Beriberi refers to a cluster of symptoms caused primarily by a nutritional deficit in vitamin B1, or thiamine. There are three types of beriberi: wet, dry, and gastrointestinal, with the classic triad of neuritic beriberi symptoms being ataxia, confusion, and ocular palsies. Because most foods are now vitamin enriched, beriberi is rare in the United States. But older adults, who have a high prevalence of chronic marginal nourishment and who are often taking diuretics, are at higher risk for thiamine deficiency. This case report demonstrates that the simple replacement of thiamine can yield remarkable results when there is a deficient state. The possibility of a symptomatic thiamine deficiency in older patients must be taken seriously and considered early on when attempting to diagnose relevant symptoms.
Key words: beriberi, thiamine, malnutrition, malnourishment, older adults
Beriberi is an archaic name for the condition caused by a deficiency of thiamine (vitamin B1). Without sufficient thiamine, there is impaired pyruvate utilization, causing increased plasma pyruvate levels and a shortage of cellular ATP. Thiamine deficiency has been suggested to be associated with neurodegenerative diseases through its role in oxidative and glucose metabolisms.1
Historically, beriberi is divided into different categories based on the primary manifestations of a thiamine deficiency. It is classified as neuritic (or “dry” beriberi) when there is a predominance of neurological manifestations such as confusion, apathy, delirium, sensory and motor neuropathy, or Wernicke/Korsakoff syndrome. A second symptom complex from thiamine deficiency relates to cardiac manifestations (or “wet” beriberi), with high output cardiac failure and eventual circulatory collapse.2 A third clinical syndrome of beriberi has recently been re-described with thiamine deficiency, causing a gastrointestinal (GI) syndrome of nausea, vomiting, abdominal pain, and lactic acidosis (ie, GI beriberi). In the 1940s, several separate experiments induced thiamine deficiency in humans; almost all participants reported nausea, vomiting, and abdominal pain.3,4 These early observations of prominent GI symptoms with thiamine deficiency appear to be have been overlooked and were never translated into a clinical category of beriberi and, thus, have essentially been forgotten.3
Clinicians are taught that there is a classic triad of symptoms associated with thiamine deficiency presenting as Wernicke/Korsakoff syndrome with ataxia, confusion, and ocular palsies. This classic presentation occurs in as few as one-third of cases of thiamine deficiency, and, in most cases, only one or two elements of the classic triad are apparent.5 Clinical records document a high incidence of mental status abnormalities (82%) but a much lower rate of ataxia (23%), ocular abnormalities (29%), and polyneuropathy (11%) in patients with documented thiamine deficiency.5-9
The misconception that thiamine deficiency is seen only in alcohol abusers is dangerous, but in clinical practice, thiamine deficiency is rarely considered in the non-alcoholic patient. In an autopsy series, a diagnosis of Wernicke’s encephalopathy pre-mortem was made in only 26 of 131 cases.10 In another 5-year autopsy series, 23% of the pathological changes of Wernicke’s encephalopathy were found in non-alcoholics.10
The most common clinical conditions associated with symptomatic thiamine deficiency are inadequate intake or excessive losses of thiamine (Table 1). Thiamine is found in larger quantities in food products such as yeast, legumes, pork, rice, and cereals. Milk products, fruits, and vegetables are poor sources of thiamine. Thiamine is denatured at high temperatures. Therefore, cooking, baking, and pasteurization can destroy thiamine.1

Another mechanism for the development of thiamine deficiency is intake of excessive amounts of thiaminase. Thiaminases are enzymes found in plants and the raw flesh and viscera of certain fish and shellfish. When ingested, these enzymes split thiamine and render it inactive.11 Thus, the ingestion of significant amounts of thiaminase can induce thiamine deficiency even though there may be a sufficient amount of thiamine in the diet. Many thiaminases are denatured by heat, but apparently vary in their heat stability.11,12,13
Another mechanism of thiamine deficiency is excessive intake of polyphenols (micronutrients with in vitro antioxidant effects) currently popularized as disease fighting and having anti-aging effects. Polyphenols are found in fruit, some plants, spices, vegetables, cereals, nuts, cocoa, dark chocolate, and in beverages such as tea and red wine and may affect intestinal and placental transport of thiamine. Chronic intake of polyphenols can convert thiamine to an unabsorbable and inactive form and theoretically can worsen or cause thiamine deficiency. However, this interaction appears to lack clinical relevance in industrialized countries, where most individuals consume adequate dietary thiamine and ascorbic acid (which prevents the polyphenol-thiamine interaction). This mechanism of thiamine deficiency has been described in Asian populations where individuals chew fermented tea leaves.14,15
In this article, we present a case report to illustrate our experience of beriberi in a 59-year-old woman with prior bariatric surgery who was malnourished from recurring nausea and vomiting. Because older adults often have inconsistent or poor diets, the possibility of a symptomatic thiamine deficiency must be taken seriously and considered early when evaluating patients.
Case Report
A 59-year-old female was admitted to our post-acute care unit for rehabilitation after a 6-day stay in an acute-care hospital. At hospital presentation, she had a 2-week history of nausea and vomiting causing weakness and an altered mental status. She also had volume contraction, causing transient acute kidney injury at the time of her admission to the hospital. Following evaluation in the hospital, she was suspected of having narcotic bowel syndrome and diabetic gastroparesis.
She stated that she had a past history of sporadic episodes of nausea and vomiting over many years, but her prior episodes had shorter duration than the current incident leading to her hospitalization. While relating her history at the post-acute care facility, she repeatedly asserted her inability to understand the severity of the weakness and debility she was experiencing.
Her family reported that she had been disoriented and hallucinating while in the hospital, but the patient had no recollection of these events. Her nausea and vomiting resolved in the hospital after tapering her opioid dose. With rehydration, her acute kidney injury resolved. She had a past medical history of two bariatric surgeries; initially, she had undergone intestinal bypass, which had been reversed within several months because of complications. She then had a second procedure with a vertical banding gastroplasty performed in the 1980s.
Upon examination at the post-acute care facility, she was obese, lethargic, and inattentive but not in distress. Her physical examination was within normal limits, with the exception of her neurologic examination. In the neurological examination, she was noted to have prominent lateral gaze nystagmus. During the examination, intermittent disconjugate gaze was observed. On confrontation, her external ocular movements appeared within normal limits. She had absence of both knee and ankle reflexes.
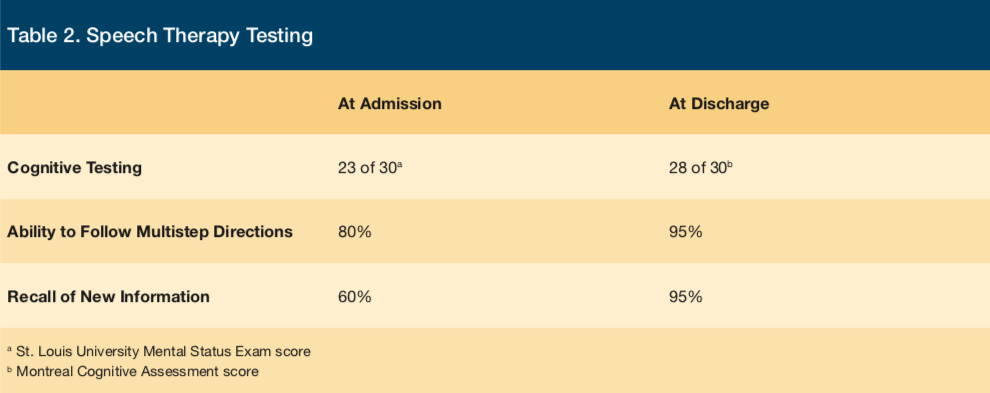
Her initial speech therapy evaluation revealed impairment in her communication and cognitive abilities, impairing both receptive and expressive language skills. She demonstrated deficient understanding of open-ended and complex questions, difficulty following multi-step directions, and poor regulation of social exchanges. Her SLUMS (St. Louis University Mental Status Exam) score was 23 of 30, demonstrating deficits in orientation, recall, and attention. At admission, her baseline recall of new information was assessed at 60% accuracy.
On initial evaluation by occupational therapy, the patient’s right-hand grip strength was 26 lbs (average, 57 lbs) and her left-hand grip strength was 12 lbs (average, 47 lbs). Her TUG (Timed Up and Go) was 57 seconds, with a score of > 15 seconds being associated with an increased risk of falling.16 Her standing functional reach was 4 inches, with a reach of less than 7 inches being indicative of an increased fall risk.17
The patient’s first physical therapy evaluation revealed significant functional deficits, initially requiring moderate assistance for transfers and minimal assistance during ambulation for maximum distance of 25 ft requiring a rolling walker. Functional testing at admission also included the 30-Second Chair Rise test, which she was unable to perform.
Upon review of the laboratory results from her hospital record, a whole blood thiamine level of 52 nmol/L (reference range, 70–180 nmol/L) was found. She had not received thiamine replacement during her hospital stay. With her indifference, inattentiveness, profound asthenia, history of bariatric surgery, recent prolonged decreased oral intake, and a blood test showing thiamine deficiency, a diagnosis of beriberi (thiamine deficiency) was made, and the patient was started on supplemental thiamine.
The patient was initially treated with intramuscular thiamine 50 mg daily for 3 days, as well as oral thiamine 200 mg daily. Within 3 days, she had a dramatic improvement in her sense of wellbeing, mental alertness, and mental focus. She realized that she was dramatically improved and readily described herself as “addled” over the preceding several days. She had minimal recollection of encounters she had during her initial 2 days in our facility (prior to the initiation of thiamine supplementation). After a 2-week rehabilitative stay, our patient’s discharge evaluation by speech therapy revealed that recall of new information was improved from an admission level of 60% accuracy to 95% accuracy. Her ability to follow multistep directions improved from a baseline of 80% accuracy to 95% accuracy. Her initial impairment in both receptive and expressive language skills had normalized, as had her cognitive deficits. She scored within normal limits (28 of 30) on the MoCA (Montreal Cognitive Assessment) (Table 2).
Her occupational therapy discharge evaluation showed that her right-hand grip strength had improved from a baseline of 26 lbs to 55 lbs, and her left-hand grip strength from a baseline of 12 lbs to 52 lbs, both of which demonstrate an improvement of one standard deviation. Her standing functional reach had improved from a baseline of 4 in to 15 in, which reflects a marked improvement in safety and reduced fall risk. Her TUG improved from a baseline of 57 seconds to 8 seconds (Table 3). For the TUG score, the approximate MDC (minimal detectable change) is 3.5 seconds.16 Therefore, her TUG score improved nearly 14-fold above the accepted MDC. She improved from requiring significant physical assistance to perform activities of daily living and self-care to being independent with her activities of daily living and self-care.

The patient made dramatic functional gains in her discharge physical therapy evaluation. She was ambulating over 300 ft without an assistive device. She improved from her baseline of being unable to rise from the chair to achieving a score of 11 stands during the 30 Second Chair Rise test at discharge. She was also able to complete all transfers and bed mobility independently (Table 4).

Discussion
This case demonstrates how important it is to maintain a high index of suspicion of symptomatic thiamine deficiency in any clinical setting with reduced dietary intake or in situations that might pose excessive losses of thiamine and not just when treating a patient with a history of alcohol abuse. Our patient did not drink alcohol and, therefore, did not have the historically expected risk factor for thiamine deficiency of alcohol abuse. However, she did have a more contemporary risk factor for vitamin deficiency because she had undergone bariatric surgery in the past and took no vitamin B supplement. Additionally, she had recently experienced a 2-week episode of nausea and vomiting.
In all patients, we must develop an increased suspicion of symptomatic thiamine deficiency whenever there has been a period of poor dietary intake. Thiamine is water-soluble and has a half-life of approximately 10-20 days due to limited tissue storage. Unless a daily intake of at least 2 mg is maintained, a deficient state can develop. Additionally, furosemide therapy may be associated with clinically significant thiamine deficiency due to increased urinary loss of water-soluble thiamine.14 Some individuals appear to develop thiamine deficiency more easily than others, indicating a suspected genetically transmitted resistance to thiamine, which renders an affected patient more susceptible to marginally-reduced thiamine intake.19 Women appear more susceptible to the development of Wernicke’s encephalopathy than men.6
When thiamine deficiency is suspected, administration of 200 to 500 mg of parenteral thiamine three times daily for 3-5 days is recommended.20, 21 This high dose of parenteral replacement is indicated due to failure of smaller doses to produce improvement in some patients with Wernicke’s encephalopathy, and, because the GI absorption of thiamine is erratic in the malnourished or alcoholic patient, parenteral replacement is recommended.20-22 Thiamine replacement should be started prior to administration of intravenous glucose, because glucose administration in the setting of thiamine deficiency can precipitate an acute Wernicke’s encephalopathy. When thiamine deficiency is suspected, it is important that supplementation should be administered without the delay of awaiting laboratory confirmation, with the response to the thiamine replacement confirming the diagnosis of a deficient state.
Thiamine deficiency should be in the differential diagnosis of all patients with delirium, confusion, weakness or lethargy, especially in the setting of marginal intake for even a relatively short period of time. Thiamine deficiency is a common condition in older adults, especially in hospitalized and institutionalized patients, and has been linked to a higher proportion of falls, Alzheimer’s disease, and depression.9 The main barrier to an accurate diagnosis is a lack of clinical suspicion in the non-alcoholic patient and an assumption that vitamin deficiencies in the modern era are rare because of the fortification of foods with vitamins. For practical purposes, all at-risk patients with undiagnosed mental status changes, oculomotor abnormalities, ataxia, unexplained delirium, or lethargy should receive parenteral thiamine replacement empirically.
Conclusion
In our frail elderly population, with a high prevalence of chronic marginal nourishment and the common exposure to diuretics that increase loss of water-soluble thiamine further, we must be at an even higher level of suspicion for the possibility of thiamine deficiency. Our patient had documented thiamine deficiency and responded dramatically to parenteral replacement, proving that her altered mental status, debility, and hallucinations had been caused by her inadequate intake of thiamine. Beriberi is an ancient term, but the syndromes associated with thiamine deficiency are still under-recognized. As this case demonstrates, simple replacement with thiamine can yield remarkable results when there is a deficient state.
1. Koh F, Charlton K, Walton K, McMahon AT. Role of dietary protein and thiamine intakes on cognitive function in healthy older people: a systematic review. Nutrients. 2015;7(4):2415-2439.
2. So, Y. Wernicke Encephalopathy. UpToDate Web site. http://www.uptodate.com/contents/wernicke-encephalopathy. Updated May 5, 2015. Accessed December 15, 2015.
3. Donnino, M. Gastrointestinal beriberi: a previously unrecognized syndrome. Ann Intern Med. 2004;141(11):898-899.
4. Williams R, Mason HL, Wilder RM, Smith BF. Observations on induced thiamine deficiency in man. Arch Intern Med. 1940;66(4):785-799.
5. Torvik A, Lindboe CF, Rogde S. Brain lesions in alcoholics. A neuropathological study with clinical correlations. J Neurol Sci. 1982;56(2-3):233-248.
6. Victor M, Adams RA, Collins GH. The Wernicke-Korsakoff syndrome and related disorders due to alcoholism and malnutrition. Philadelphia, PA: FA Davis; 1989.
7. Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49(4):341-345.
8. O’Keeffe ST, Tormey WP, Glasgow R, Lavan JN. Thiamine deficiency in hospitalized elderly patients. Gerontology. 1994;40(1):18-24.
9. Pepersack T, Garbusinski J, Robberecht J, Beyer I, Willems D, Fuss M. Clinical relevance of thiamine status amongst hospitalized elderly patients. Gerontology. 1999;45(2):96-101.
10. Lindboe CF, Løberg EM. Wernicke’s encephalopathy in non-alcoholics. An autopsy study. J Neurol Sci. 1989;90(2):125-129.
11. Studdert VP, Gay CC, Blood DC. thiaminase. Saunders Comprehensive Veterinary Dictionary 3rd ed. 2007. http://medical-dictionary.thefreedictionary.com/thiaminase. Accessed December 15, 2015.
12. Aparicio Vizuete A, Robles F, Rodríguez-Rodríguez E, López-Sobaler AM, Ortega RM. Association between food and nutrient intakes and cognitive capacity in a group of institutionalized elderly people. Eur J Nutr. 2010;49(5):293-300.
13. Cornell University College of Agricultural and Life Sciences. Plants Poisonous to Livestock. Cornell University Web site. http://poisonousplants.ansci.cornell.edu/toxicagents/thiaminase.html. Updated September 10, 2015. Accessed December 15, 2015.
14. Martel F1, Monteiro R, Calhau C. Effect of polyphenols on the intestinal and placental transport of some bioactive compounds. Nutr Res Rev. 2010;23(1):47-64.
15. Manach CC, Scalbert A, Morand C, Rémésy C, Jiménez L. Effect of polyphenols on the intestinal and placental transport of some bioactive compounds. Am J Clin Nutr. 2004;79(5):727-747.
16. Huang, SL, Hsieh CL, Wu RM, Tai CH, Lin CH, Lu WS. Minimal detectable change of the timed “up & go” test and the dynamic gait index in people with Parkinson disease. Phys Ther. 2011;91(1):114-121.
17. Thomas JI, Lane JV. A pilot study to explore the predictive validity of four measures of falls risk in frail elderly patients. Arch Phys Med Rehabil. 2005;86(8):1636-1640.
18. Seligmann H, Halkin H, Rauchfleisch S, et al. Thiamine deficiency in patients with congestive heart failure receiving long-term furosemide therapy: a pilot study. Am J Med. 1991;91(2):151-155.
19. Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27(2):134-142.
20. Agabio R. Thiamine administration in alcohol-dependent patients. Alcohol Alcohol. 2005;40(2):155-156.
21. Cook CC, Hallwood PM, Thomson AD. B Vitamin deficiency and neuropsychiatric syndromes in alcohol misuse. Alcohol Alcohol. 1998;33(4):317-336.
22. Day E, Bentham P, Callaghan R, Kuruvilla T, George S. Thiamine for Wernicke-Korsakoff Syndrome in people at risk from alcohol abuse. Cochrane Database Syst Rev. 2004;1:CD004033.










