Assessing Skin for Pressure Damage: Review of the Revised Section M of the MDS 3.0
The significant expansion of Section M goals in Version 3.0 of the Centers for Medicare & Medicaid Services (CMS) Minimum Data Set (MDS 3.0)1 reflects the increased clinical relevance of this assessment. Section M: Skin Conditions requires the assessor to address actual and potential skin concerns and certain skin treatments. The Resident Assessment Instrument (RAI) User’s Manual2 includes very specific directions and coding clarifications to ensure that the clinician provides an accurate clinical picture of the resident’s condition on the MDS 3.0.
For Section M of the MDS 3.0, CMS has adapted clinical staging definitions from the National Pressure Ulcer Advisory Panel (NPUAP).3 Clinicians are now required to code each pressure ulcer according to its deepest anatomical stage until said ulcer has healed; back-staging or reverse staging—permitted under MDS 2.0—is no longer allowed (ie, a stage 3 ulcer is to be coded as a stage 3 ulcer until healed). Clinicians are no longer required to stage ulcers other than pressure ulcers, but they must report the most severe tissue type associated with a particular pressure ulcer. If a pressure ulcer is stage 3 or 4 or unstageable (due to slough or eschar), its measurements must be reported. Diabetic ulcers are also now included in Section M.
Prior to proceeding with Section M coding and, more importantly, with wound treatment, wound etiology must be determined. CMS notes that if a wound has multiple causes, the primary cause must be established so that the wound can be classified appropriately.
Although the coding instructions and clarifications in the RAI User’s Manual are very clear, numerous clinical practice issues can affect the accuracy of MDS 3.0 reporting, and a thorough review of facility practices is warranted. Skin care practices, including pressure ulcer prevention and management, relevant policies and procedures, and documentation (eg, assessment) tools should be reviewed in full consideration of the guidance provided by the RAI User’s Manual. Facility leadership should ensure that physicians and other medical providers are educated regarding Section M and other sections of the MDS 3.0. We discuss a few key MDS 3.0 items from Section M and related policy and educational considerations.
Assessing Pressure Ulcer Risk
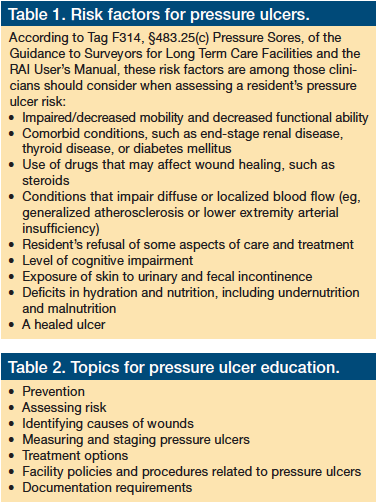
Recognizing the critical importance of risk assessment, CMS begins Section M by asking the assessor to indicate the methods used to establish whether the resident is at risk for developing pressure ulcers. In addition to providing the assessor with an opportunity to note if the resident has a pressure ulcer ≥stage 1, a scar over a bony prominence, or a nonremovable dressing or device, the assessor is asked to indicate whether a formal assessment (ie, use of an established assessment tool) was completed and if the resident’s estimated level of risk was based on a clinical assessment. A comprehensive clinical risk assessment should include a review of the medical record and a head-to-toe assessment of the resident (Table 1).4
Some risk factors listed in Table 1, along with some risk factors that are not on the list, are not fully modifiable. Facility staff must be sure to note all risk factors on the plan of care, regardless of whether they are addressed in the formal risk assessment tool used by the clinician or the MDS 3.0, and staff must also document planned approaches for handling any risks identified. On page 4-10 of the RAI User’s Manual, CMS reminds the assessor that the plan of care should be oriented to “managing risk factors to the extent possible or indicating the limits of such interventions.” It is essential to educate the staff—including the direct care staff—on methods that can be used to identify a resident’s risk factors for pressure ulcer development and on steps that can be taken to reduce or eliminate a patient’s risk.
Staging Pressure Ulcers
Section M requires that the clinician document the stage of any pressure ulcer identified and, for all ulcers >stage 1, indicate whether the ulcer was present at admission. The assessor must also note the date of the oldest stage 2 pressure ulcer. As part of the assessment, clinicians should attempt to determine the date of origin for any pressure ulcers. When they cannot determine the date of the oldest stage 2 pressure ulcer, they should use dashes to fill in this MDS 3.0 item, which signifies that the information is not available.
CMS notes in the RAI User’s Manual that the agency adapted the staging definitions for pressure ulcers in MDS 3.0 from the NPUAP’s definitions and advises facilities to take special care to code the MDS according to the instructions in the RAI User’s Manual and not per NPUAP staging guidance. If a facility is using the NPUAP staging system to indicate the status of pressure ulcers, it might be prudent to include clarifying information in the medical record, as needed, that supports MDS 3.0 coding.
To ensure that pressure ulcers are documented accurately, it is critical to educate responsible staff members on how to stage them appropriately and then validate their competency at doing so. Nurses at long-term care facilities often voice concern regarding discrepancies among clinicians in how they document the stage of ulcers in a resident’s medical record. Staff should act swiftly to resolve such discrepancies in the medical record and ensure that it accurately reflects the resident’s clinical picture.
The item in Section M that seems to have spurred the most discussion is “M0610: Dimensions of Unhealed Stage 3 or 4 Pressure Ulcers or Unstageable Pressure Ulcer Due to Slough or Eschar.” This MDS item asks the assessor for the dimensions of the stage 3, 4, or unstageable (due to slough or eschar) pressure ulcer with the largest surface area (ie, length x width). According to the RAI User’s Manual, the ulcer’s dimensions, including its length (longest measurement head-to-toe), width (widest point perpendicular to length), and depth (deepest point of the ulcer), should be documented in centimeters to the nearest tenth. When nurses responsible for completing the MDS are asked about the definitions their facility policy uses to measure these dimensions, many are unsure and some do not even know whether their facility has a policy that defines the terms. When asked about their individual practice, most nurses do not recall any instance since the MDS 3.0 was implemented in which they documented a pressure ulcer in which width exceeded its length. It is possible, but highly unlikely, that all ulcers in a given facility would have a head-to-toe measurement longer than the dimension perpendicular to it. It is more likely that the nurse is indicating the wound’s longest measurement as its length, regardless of the orientation of the wound on the resident’s body.
Measurement protocols need to be reviewed and revised, if necessary, to ensure that they comply with the MDS 3.0 coding instructions. Nurses engaged in wound measurement should receive routine instruction on techniques for assessing and measuring wounds, and their competency should be observed.
Conclusion
Appropriate and effective skin care, which includes prevention and treatment of pressure ulcers, begins with assessing the resident thoroughly and accurately. In the RAI User’s Manual, CMS stresses the need for taking a holistic approach when evaluating a resident’s skin status. Staff members need to be educated on the best practices for pressure ulcer prevention, assessment, and management, and should be prepared to use them (Table 2). With accurate assessment techniques and a holistic, interdisciplinary approach to the prevention and management of pressure ulcers and other skin problems, excellence can be achieved.
The author is employed by Harmony Healthcare International, which provides MDS consulting.
Ms. Pettis is director of program development, Harmony Healthcare International, Topsfield, MA. She is also an active member of the American Association for Long Term Care Nursing (AALTCN).
References
1. Saliba D, Buchanan J. Development & validation of a revised nursing home assessment tool: MDS 3.0. https://www.cms.gov/nursinghomequalityinits/25_nhqimds30.asp. Published April 2008. Accessed August 31, 2011.
2. Centers for Medicare & Medicaid Services. Nursing Home Quality Initiatives. https://www.cms.gov/NursingHomeQualityInits/45_NHQIMDS30TrainingMaterials.asp. Published October 2011. Accessed September 19, 2011.
3. National Pressure Ulcer Advisory Panel. Pressure Ulcer Stages Revised by NPUAP. www.npuap.org/pr2.htm. Published 2007. Accessed September 19, 2011.
4. Centers for Medicare & Medicaid Services. State operations manual: Appendix PP - Guidance to surveyors for long term care facilities. https://www.cms.gov/manuals/downloads/som107ap_pp_guidelines_ltcf.pdf. Revised January 7, 2011. Accessed August 31, 2011.










