Applications of Nonpharmacological Management of Chronic Insomnia in Long-Term Care
Poor sleep and insomnia are common among older adults, including those living in long-term care (LTC) settings. Current pharmacotherapeutic interventions for insomnia disorders have not been evaluated in LTC settings, and recent recommendations suggest that their use should be avoided whenever possible. Non-pharmacological interventions have been extensively studied in older adults, and many can be translated for use in LTC settings. Effective interventions include programs based on cognitive-behavioral therapy for insomnia (CBTI) principles; interventions targeting circadian rhythms, such as timed exposure to bright light; and complementary and alternative interventions, such as yoga and tai chi.
Key words: sleep, insomnia, aging, cognitive-behavioral therapy, long-term care
Insomnia disorder refers to dissatisfaction with sleep quantity or quality (ie, difficulty initiating sleep, difficulty maintaining sleep, or waking up too early) and associated deficits in daytime function (eg, fatigue, memory impairment, daytime sleepiness, and impaired social performance). According to the 2014 International Classification of Sleep Disorders, 3rd edition, these symptoms must occur at least three times per week, should last for 3 months or longer, and must not be due entirely to an inadequate opportunity for sleep, an inappropriate sleep environment, or another sleep disorder.1 Some of the negative consequences of chronic insomnia in older adults (ie, those aged 65 years and older) are increased depressive symptoms, poor quality of life, increased morbidity (eg, cognitive impairment and hypertension), and increased mortality.2-4
Chronic Insomnia in Long-Term Care
Whereas insomnia has been studied in community-dwelling older adults, less is known about insomnia in older adults living in long-term care (LTC) settings. Broadly, LTC encompasses environments in which medical and nonmedical services are provided to individuals with chronic illnesses or disability who cannot care for themselves for long periods of time.5 Most of the information available about insomnia in LTC residents comes from observational studies performed in nursing homes (NHs) and assisted living facilities (ALFs). A recent longitudinal study carried out in multiple NHs from eight countries found that 24% of the residents had difficulty falling asleep or staying asleep, woke up too early, or had non-restorative sleep.6 Among NH residents, a 1-year increase in age is associated with a 1% increase in the risk of insomnia.6 Moreover, among NH residents, insomnia is associated with several adverse health outcomes; for instance, disruptive behaviors and psychological distress, low levels of activities and social engagement, and high levels of communication difficulties and interpersonal conflicts.7,8 Residents of ALFs frequently report dissatisfaction with the quality of sleep and the consequences of this lack of quality. An observational study9 that used the Pittsburgh Sleep Quality Index (also known as PSQI, a validated, 18-item, self-reported sleep quality questionnaire10), found that 65% of residents from 18 ALFs had clinically significant sleep disturbance (PSQI score >5). The same study showed that greater sleep disturbance was associated with declining health-related quality of life and worsening depressive symptoms over time.9 Because of the association of insomnia with important clinical outcomes in LTC, it is imperative to educate healthcare providers about the mechanisms that underlie insomnia as well as safe and effective treatments for older adults in these settings.
Insomnia Etiology
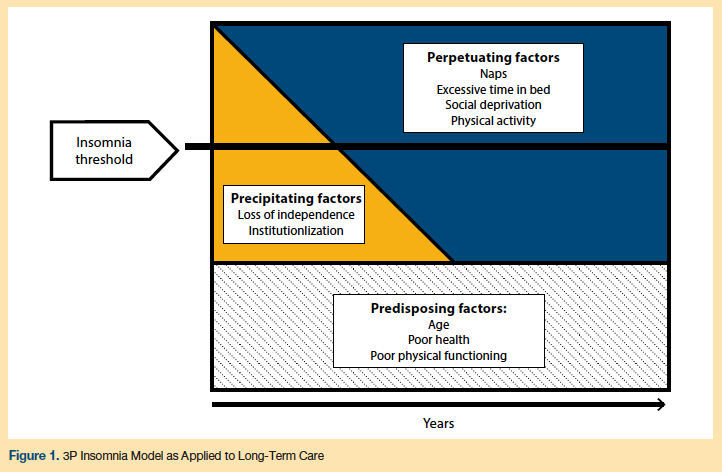
A useful conceptual model for understanding insomnia and its high prevalence across diverse settings of care, including LTC, is the “3P model” (see Figure 1).11 The first “P” refers to predisposing factors, which increase an older adult’s likelihood of experiencing insomnia. Likely predisposing factors for insomnia among LTC patients are age, poor health (eg, chronic pain, depression), and poor physical functioning.12 The second “P” represents precipitating factors, which are associated with the onset of an insomnia disorder. Common precipitating factors in older adults include hospitalizations, changes in health status, retirement from full-time work, or loss of a loved one.12 Among LTC patients, loss of independence and moving into a NH or ALF facility are particularly relevant precipitating factors. The final “P” of the model accounts for perpetuating factors, which sustain insomnia. Most of these perpetuating factors are behaviors such as taking naps and spending too much time in bed.12 These behaviors are generally adopted by individuals to compensate for the lack of restorative sleep; however, in the long-term, these behaviors perpetuate impaired sleep. In addition to these common perpetuating factors, patients in the LTC setting are exposed to a unique set of perpetuating factors that include frequent nighttime caregiving, lack of daytime social interaction, physical inactivity, inadequate light exposure during the day, and a sleep environment that is too noisy and bright.13 Applying the 3P model for insomnia in LTC can guide adequate interventions for LTC residents with insomnia.
Pharmacological Treatment for Chronic Insomnia
Hypnotic or sedative medications are widely used in LTC patients with impaired sleep;14 however, these drugs do not target the etiological and perpetuating factors that underlie chronic insomnia and cause it to be maintained over time. Several meta-analyses have concluded that hypnotics may have a modest benefit in terms of improving sleep quality of older adults; nevertheless, the incidence of negative side effects makes hypnotic drugs a risky choice for insomnia treatment in older adults.15,16 A number of recent clinical guidelines and statements have expressly recommended against the long-term use of hypnotic medications for older adults.17,18 The vast majority of pharmacological interventions have been tested in community-dwelling individuals, rather than those living in LTC settings, and very few studies have been carried out with adults aged 65 years or older. The available information about hypnotics in older adults with functional limitations or comorbid medical conditions is limited. Studies do show, however, that use of hypnotic drugs is associated with more dependence in activities of daily living, excessive sedation, cognitive impairment, agitation, delirium, and falls. Consequently, pharmacological interventions may be extremely deleterious in LTC patients.16
Nonpharmacological Approaches to Chronic Insomnia
Because of the complications and challenges associated with pharmacotherapy for chronic insomnia in older adults, nonpharmacological approaches are generally preferred and have demonstrated benefits. Nonpharmacological approaches to chronic insomnia treatment can be roughly grouped into three types: (1) cognitive-behavioral therapy (CBT)–based insomnia treatments; (2) interventions targeting circadian rhythms (eg, bright light exposure); and (3) complementary and alternative medicine-based treatment approaches. Although the strength of the empirical evidence varies among these nonpharmacological treatment approaches, they should generally be considered as favorable alternatives to pharmacotherapy due to their limited known harmful side effects or interactions and their superior effectiveness when compared with pharmacotherapies in head-to-head clinical trials.19,20
Cognitive-Behavioral Therapy–Based Insomnia Treatments
CBT is a mode of psychotherapy that focuses on the relationships between thoughts, behaviors, and feelings. CBT-based insomnia treatments (commonly referred to as CBTI) include a combination of cognitive-based and behavior-based treatment strategies, grouped into multicomponent packages,21 and are based on the general principles and practices of CBT. Symptom reduction is typically achieved through monitoring thoughts and directly intervening on these thoughts and on behaviors, with the goal of modulating one’s feelings and emotions. CBTI includes: sleep education, cognitive therapy, sleep hygiene, relaxation strategies, stimulus control, and sleep restriction or compression. Sleep education consists of providing the older adult with insomnia information on factors governing biological sleep need and sleep timing as well as information on how sleep is regulated. Cognitive therapy involves the identification and alteration of dysfunctional beliefs and attitudes about sleep. Sleep hygiene consists of basic rules to avoid sleep disrupting substances, behaviors, and environmental factors (eg, caffeine, TV in bed, noisy sleep environment). Relaxation strategies involve any number of techniques (eg, progressive muscle relaxation, meditation, autogenic techniques, mental imagery) aimed at calming the body and/or mind. Stimulus control is a behavioral strategy that aims to increase the response of sleep to the stimulus of the bed and bedroom. Sleep restriction and compression are behavioral strategies with the goal of closely aligning time in bed to actual time spent sleeping. Reducing the amount of time an individual spends in bed increases the amount of time that they have been awake during the day. This yields an increase in “sleep pressure,” which increases the likelihood that, once in bed, the patient will be able to sleep throughout the night.
CBTI has demonstrated medium-to-large effects in improving the sleep of older adults.22,23 It is considered the optimal first-line treatment for insomnia in adults of any age.24-26
CBTI interventions require some adaptations for use in LTC settings. Given the myriad contextual and environmental factors beyond the control of any individual LTC resident (eg, disruptions due to nursing care of residents, disruptive behaviors of other residents), the magnitude of any effects may be attenuated. However, a number of the individual strategies included in CBTI are believed to still be appropriate intervention strategies in LTC settings. The most potent CBTI strategies for improving the sleep of elders in LTC facilities are likely to include: sleep hygiene (specifically, improvement of the sleep environment), stimulus control, sleep restriction, and relaxation strategies.
As an example of how CBTI may be adapted, the traditional instruction for stimulus control involves asking patients to remove themselves from bed during the night if they experience prolonged periods of wakefulness. In the LTC setting, this recommendation may not be safe or feasible. An appropriate adaptation to this recommendation may be to provide residents with alternative activities that can be done safely within one’s bed during the night (eg, relaxation music or reading materials). This intervention could be easily implemented and would serve to minimize frustration associated with wakefulness in bed.
One behavioral component of CBTI that is likely to have a large impact in LTC facilities is the implementation of sleep restriction therapy, which centers on avoidance of extended daytime napping. This leads to a high sleep drive at night and therefore an increased propensity to fall asleep quickly and sleep throughout the night. Given the high rates of impaired physical and mental functioning of LTC residents, staff engagement in this recommendation is necessary. Adapting this common sleep restriction technique to include staff engagement could include educating staff on the importance of avoiding daytime napping and having staff offer an increased amount of daytime activities in which residents can engage. Preliminary evidence suggests that CBTI can improve sleep quality in institutionalized older adults.27 However, more research is needed in the application of CBTI to LTC settings.
A key patient-level limitation of CBTI is that many residents suffer from cognitive impairment and therefore cannot fully engage in implementation of behavioral principles or cognitive strategies. As a result, investigators have attempted to address poor sleep using multi-component interventions that incorporate a number of CBTI principles and involve staff engagement. To date, these interventions have not provided compelling evidence regarding their effectiveness for improving sleep in LTC patients.28,29 Facility-level barriers also create challenges for improving sleep with these approaches; in particular, it is difficult to reduce nighttime noise in institutional settings. A simple strategy to potentially overcome some of these facility-level barriers is to provide residents with eye masks and earplugs. Such an intervention would reduce nighttime awakenings resulting from staff behaviors and facility factors.
Circadian Interventions
Circadian interventions involve direct exposure to carefully timed naturally occurring sunlight or bright artificial light, with the goal of strengthening an individual’s circadian rhythm to improve nighttime sleep. The rationale for this approach is that light exposure is the strongest cue for the human circadian clock. In the general older adult population with insomnia, circadian interventions have not demonstrated a reliable ability to improve the nighttime sleep of older adults.30 However, due to the documented low levels of light within LTC facilities,31 light exposure has been the focus of several clinical trials. These studies have investigated the nighttime sleep effects of increased daytime light exposure in LTC facilities. Generally speaking, interventions have resulted in consistent but small improvements in sleep.32 Additional research is needed to determine the optimal approach to bright light interventions in LTC settings. Potential approaches to increasing bright light exposure in LTC residents include: opening window covers early after awakening, spending time each morning outside, and use of bright light boxes in common areas and resident rooms throughout the day.
Complementary and Alternative Medicine (CAM) Approaches
Complementary and alternative medicine (CAM) is a broad term that generally indicates a treatment approach that includes components that are non-mainstream. These approaches can be coupled with more conventional approaches (ie, complementary medicine) used in place of mainstream approaches (ie, alternative medicine). In the United States, roughly 40% of the population uses some form of CAM in their healthcare regime. This number climbs to over 50% among individuals over the age of 65 years.33 Importantly, CAM therapies are rarely discussed with healthcare providers. Many are costly and some carry a risk of unforeseen complications. In the general older adult population, many CAM-based insomnia treatments have been tested. These treatments include: tai chi, acupuncture, yoga, and natural supplements (eg, valerian root, I-tryptophan, chamomile, etc).
The evidence supporting CAM therapies for insomnia treatment is limited and mixed. Comprehensive reviews have revealed little support for nonprescription/natural herbal products in the treatment of insomnia.34,35 However, similar empirical reviews have revealed promising preliminary support for acupuncture, tai chi, and yoga,35 with fewer studies being conducted exclusively with older adult samples.33 Research examining CAM-based therapies for insomnia in older adults in LTC facilities is very limited. We see some promise for these treatment approaches in LTC facilities, in which the concerns for overmedication are high. Tai chi, acupuncture, and yoga may be low-impact interventions that lend themselves nicely to adaptation for use in older adult groups. For example, there are successful adaptations of tai chi and yoga so that these interventions can be done while seated. Future research should examine the potential utility of CAM-based interventions for improving sleep in LTC patients.
Conclusions
Older adults frequently suffer from poor sleep. Individuals receiving LTC can have poor sleep due both to patient-level factors and institutional environments. There are concerns about use of medications for insomnia in older adults, and recent recommendations suggest their use should be avoided whenever possible. There are several nonpharmacological intervention approaches that may be beneficial in LTC. This includes programs based on cognitive-behavioral therapy for insomnia (CBTI) principles, interventions targeting circadian rhythms such as timed exposure to bright light, and complementary/alternative treatments such as yoga and tai chi. Though we offer several potential adaptations to traditional CBTI strategies, additional research to establish how best to implement treatment of insomnia into LTC settings is needed.
1. American Academy of Sleep Medicine (AASM). International Classification of Sleep Disorders, 3rd ed. Darien, IL: ASSM; 2014. aasmnet.org/library/default.aspx?id=9. Accessed November 11, 2015.
2. Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract. 2002;51(3):229-235.
3. Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491-497.
4. Parthasarathy S, Vasquez MM, Halonen M, et al. Persistent insomnia is associated with mortality risk. Am J Med. 2015;128(3):268-275.
5. Ageing and Health Programme. Towards an International Consensus on Policy For Long-Term Care Of The Ageing. World Health Organization (WHO) Website. http://www.who.int/ageing/publications/long_term_care/en/. Published 2000. Accessed November 11, 2015.
6. Gindin J, Shochat T, Chetrit A, et al. Insomnia in long-term care facilities: a comparison of seven European countries and Israel: the Services and Health for Elderly in Long TERm care study. J Am Geriatr Soc. 2014;62(11):2033-2039.
7. Voyer P, Verreault R, Mengue PN, Morin CM. Prevalence of insomnia and its associated factors in elderly long-term care residents. Arch Gerontol Geriatr. 2006;42(1):1-20.
8. Garms-Homolovà V, Flick U, Röhnsch G. Sleep disorders and activities in long term care facilities--a vicious cycle? J Health Psychol. 2010;15(5):744-754.
9. Martin JL, Fiorentino L, Jouldjian S, Josephson KR, Alessi CA. Sleep quality in residents of assisted living facilities: effect on quality of life, functional status, and depression. J Am Geriatr Soc. 2010;58(5):829-836.
10. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry
Res. 1989;28(2):193-213.
11. Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10(4):541-553.
12. Alessi CA, Schnelle JF. Approach to sleep disorders in the nursing home setting. Sleep Med Rev. 2000;4(1):45-56.
13. Schnelle JF, Cruise PA, Alessi CA, Ludlow K, al-Samarrai NR, Ouslander JG. Sleep hygiene in physically dependent nursing home residents: behavioral and environmental intervention implications. Sleep. 1998;21(5):515-523.
14. Conn DK, Madan R. Use of sleep-promoting medications in nursing home residents: risks versus benefits. Drugs Aging. 2006;23(4):271-287.
15. Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162(2):225-233.
16. Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22(9):1335-1350.
17. Campanelli CM. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
18. Pretorius RW, Gataric G, Swedlund SK, Miller JR. Reducing The Risk of Adverse Drug Events in Older Adults. Am Fam Physician. 2013;87(5):331-336.
19. Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late life insomnia: a randomized controlled trial. JAMA. 1999;281(11):991-999.
20. Sivertsen B, Omvik S, Pallesen S, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA. 2006;295(24):2851-2858.
21. Dzierzewski JM, O’Brien EM, Kay D, McCrae CS. Tackling sleeplessness: psychological treatment options for insomnia in older adults. Nat Sci Sleep. 2010;2:47-61.
22. Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25(1):3-14.
23. Pallesen S, Nordhus IH, Kvale G. Nonpharmacological interventions for insomnia in older adults: A meta-analysis of treatment efficacy. Psychotherapy Theory Research Practice Training. 1998;35(4):472-482.
24. McCurry SM, Logsdon RG, Teri L, Vitiello MV. Evidence-based psychological treatments for insomnia in older adults. Psychol Aging. 2007;22(1):18-27.
25. Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine Report. Sleep. 2006;29(11):1415-1419.
26. Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical Guideline for the Evaluation and Management of Chronic Insomnia in Adults. J Clin Sleep Med. 2008;4(5):487-504.
27. El Kady HM, Ibrahim HK, Mohamed SG. Cognitive behavioral therapy for institutionalized elders complaining of sleep disturbance in Alexandria, Egypt. Sleep Breath. 2012;16(4):1173-1180.
28. Alessi CA, Martin JL, Webber AP, Cynthia Kim E, Harker JO, Josephson KR. Randomized, controlled trial of a nonpharmacological intervention to improve abnormal sleep/wake patterns in nursing home residents. J Am Geriatr Soc. 2005;53(5):803-810.
29. Ouslander JG, Connell BR, Bliwise DL, Endeshaw Y, Griffiths P, Schnelle JF. A nonpharmacological intervention to improve sleep in nursing home patients: results of a controlled clinical trial. J Am Geriatr Soc. 2006;54(1):38-47.
30. Friedman L, Zeitzer JM, Kushida C, et al. Scheduled bright light for treatment of insomnia in older adults. J Am Geriatr Soc. 2009;57(3):441-452.
31. Shochat T, Martin J, Marler M, Ancoli-Israel S. Illumination levels in nursing home patients: effects on sleep and activity rhythms. J Sleep Res. 2000;9(4):373-379.
32. Martin JL, Ancoli-Israel S. Sleep disturbances in long-term care. Clin Geriatr Med. 2008;24(1):39-50,vi.
33. Gooneratne NS. Complimentary and alternative medicine for sleep disturbances in older adults. Clin Geriatr Med. 2008;24(1):121-138,viii.
34. Meolie AL, Rosen C, Kristo D, et al. Oral nonprescription treatment for insomnia: an evaluation of products with limited evidence. J Clin Sleep Med. 2005;1(2):173-187.
35. Sarris J, Byrne GJ. A systematic review of insomnia and complementary medicine. Sleep Med Rev. 2011;15(2):99-106.










