Antithrombotic Therapy for Atrial Fibrillation: An Update on Safety, Evidence-Based Treatment Decisions, and the New Oral Anticoagulants
Atrial fibrillation is common among frail elders in long-term care (LTC) and is a significant risk factor for severe stroke. This risk can be reduced with antithrombotic therapy, but the risk of bleeding complications from these treatments may in some cases outweigh their stroke-reduction benefits. Therefore, the decision to initiate antithrombotic therapy should be made after each patient’s benefits and risks are systematically estimated and discussed with the patient and his or her family so that a shared decision is reached. If the benefits of antithrombotic prophylaxis are judged to outweigh the risks, the clinician must then consider each patient’s comorbidities and preferences when deciding which agent to prescribe. Compared with warfarin, new oral antithrombotic agents are associated with potentially improved clinical outcomes, have better safety profiles, and are more convenient for patients and clinicians. However, clinical trials evaluating these agents excluded patients with conditions and risk factors common among patients residing in LTC settings, suggesting cautious consideration is warranted before prescribing these agents to this population. Regardless of which antithrombotic agent is chosen for a particular patient, facilities should closely monitor patients and employ policies and procedures to ensure its safe use. This article provides a review of the safety of various antithrombotic agents and how LTC providers can make evidence-based treatment decisions when prescribing these agents.
Key words: Stroke, atrial fibrillation, antithrombotic agents, warfarin, dabigatran, rivaroxaban, apixaban, long-term care, stroke risk reduction, bleeding complications.
_______________________________________________________________________________________________________________________________________________
The new oral anticoagulants dabigatran, rivaroxaban, and apixaban vie to replace warfarin as the gold standard for antithrombotic stroke prophylaxis for patients with atrial fibrillation. Although these agents tout better safety profiles, practitioners should remember the importance of conducting risk-benefit assessments, as they would when considering warfarin, before deciding whether anticoagulation and the use of any of these newer anticoagulants is appropriate for frail long-term care (LTC) patients with multiple comorbid conditions. This cautious approach recognizes that, in older LTC residents, the rates of both adverse and beneficial outcomes seen with new oral anticoagulants may be similar to those observed with warfarin. Before prescribing any newer anticoagulants to LTC residents, prudent practitioners should consider several factors, including the comparability of the study populations in the clinical trials of these agents with their LTC patients; the cost, convenience, and safety of these agents; and the medication benefits and harms, as determined using clinically relevant calculations, such as absolute risk reduction (ARR), absolute risk increase (ARI), number needed to treat (NNT), or number needed to harm (NNH). This article summarizes recent advances in risk-benefit assessments for anticoagulation in persons with atrial fibrillation, addresses new recommendations for special subpopulations of patients with atrial fibrillation, and reviews recent trials of new oral anticoagulants.
Atrial Fibrillation Overview
Atrial fibrillation is the most common sustained arrhythmia,1 affecting approximately 2.3 million people in the United States.2 The prevalence of atrial fibrillation increases steadily with age, from 0.5% among those aged 50 to 59 years to 8.8% among those aged 80 to 89 years.3 If left untreated, atrial fibrillation can lead to heart failure, but its most devastating complication is also its most imminent threat: stroke. Atrial fibrillation increases the risk of stroke 4- to 5-fold.1 In addition, the risk of stroke that is independently attributable to atrial fibrillation increases with age, rising from approximately 1.5% for those aged 50 to 59 years to 23.5% for those aged 80 to 89 years.4
Risks and Benefits of Anticoagulation for Stroke Prevention in Atrial Fibrillation
When warfarin is compared with placebo for stroke prophylaxis in atrial fibrillation, it reduces the risk of stroke by 66%, representing an NNT of 32, and it reduces death by 25%, representing an NNT of 43.1,5 In addition, when a stroke occurs while a patient is taking warfarin, the likelihood of severe disability resulting from the stroke is reduced.6 These benefits of oral anticoagulation are often discounted by clinicians, whose decisions are influenced by an overestimation of the risk of bleeding complications, especially subdural and intracerebral bleeding.7 In addition, clinicians often overestimate the small additional attributable risk of developing a subdural hematoma following a fall, particularly among persons prone to falling.7 Despite warfarin being associated with an increased number of adverse effects, and these adverse effects increasing with advancing age and comorbidities, it is precisely the patients with the highest risk for complications who can reap the highest net clinical benefit from anticoagulation therapy with warfarin.8
Numerous complexities are involved when deciding to start or continue anticoagulation for a frail LTC patient, and these complexities do not lend themselves to simple heuristics like “too old for anticoagulation” or “high risk for falls.”9 Ideally, the clinician should engage the patient and/or family in a shared decision-making process that uses decision-support tools and clinical evidence to determine which antithrombotic treatment plan best addresses each patient’s unique set of comorbidities, goals, preferences, and values.10,11
Antithrombotic Therapy Risks and Benefits
A stepwise approach can help clinicians assess the risks and benefits of antithrombotic therapy and optimize safety (Table 1). The first step in this process is for the clinician to assess the patient’s condition and prognosis and assess whether anticoagulation is likely to improve his or her quality of life or length of life. Thereafter, the clinician should estimate the patient’s risk of stroke using an instrument such as the CHADS2 tool12; however, one of the limitations of this tool is that it does not incorporate the increasing risk of stroke attributed to advancing age. Therefore, LTC providers need to keep in mind that it may underestimate stroke risk in their elderly patients. Using the tool, clinicians can then calculate an individual’s expected benefit from antithrombotic prophylaxis for atrial fibrillation. For example, oral anticoagulant therapy would be expected to reduce stroke risk by 66% for patients with a CHADS2 score of 3, from a risk of 6% to 2% annually.5 For the population of patients with atrial fibrillation, the American College of Chest Physicians (ACCP) calculates an expected reduction in all-cause mortality of 25% to
between 5.3% and 3.9%.5
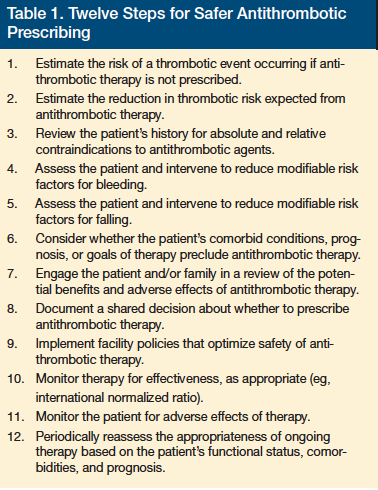
Bleeding Risk
Before initiating antithrombotic therapy, clinicians need to quantify their patients’ bleeding risk. Many bleeding risk factors are also risk factors for stroke. Bleeding risk can be estimated using clinical tools, such as the HAS-BLED score, or by referring to pooled data from research studies.13,14 However, current bleeding risk scores are limited by modest predictive accuracy and absence of data for new oral anticoagulants.5 The estimated rate of nonfatal extracranial bleeding in a patient receiving warfarin therapy is 1.3% per year, compared with a baseline bleeding risk of 0.5% when not on therapy, serving as a reminder that 38% of bleeding events during warfarin therapy may not be directly attributable to warfarin alone.5 The ACCP guidelines for atrial fibrillation estimated fatality rates of 15% for extracranial bleeding, 50% for hemorrhagic stroke, and 25% for ischemic stroke.5
Ideally, clinicians should incorporate individual patient values into the shared decision-making process, balancing potential bleeding risks with the potential benefits expected from antithrombotic therapy. The recently published ACCP guideline for antithrombotic stroke prophylaxis for atrial fibrillation included consideration of patient preferences and employed rigorous methods to re-analyze and compare antithrombotic research studies.5,15 For example, citing patient perceptions that rated a stroke as an outcome three times more undesirable than a nonfatal extracranial bleeding event, the ACCP endorsed only those treatments proven to prevent three or more strokes for every major bleeding event.5
To reduce the risk of bleeding events among patients receiving antithrombotic agents, clinicians should identify and minimize modifiable risk factors for bleeding and falls. Gastrointestinal bleeding risk can be reduced by limiting the use of nonsteroidal anti-inflammatory drugs and dual antiplatelet therapy, or by adding gastric cytoprotection with a proton pump inhibitor for high-risk patients. Clinicians can mitigate fall risk by reducing polypharmacy, optimizing environmental safety, encouraging physical activity to improve muscle strength and balance, and supplementing with vitamin D, particularly for elders found to have low serum vitamin D concentrations.
Patients and clinicians alike fear the serious and potentially fatal conditions of subdural or intracranial hemorrhage, whether due to intrinsic brain pathology, such as cerebral amyloid angiopathy; adverse medication effects from excessive anticoagulation; or trauma from a fall. All of these are legitimate concerns, but with regard to falls, it is important for clinicians to be aware that the estimated attributable risk for developing a subdural hematoma following a fall is fairly low at approximately one or two additional subdural hematomas per 10,000 elderly patients who fall.7
Monitoring and Safety Policies
Once an antithrombotic treatment plan has been selected, LTC facilities must employ policies and procedures to ensure safe use among and monitoring of patients receiving antithrombotic medications. Methods to enhance safety include the following: using approved algorithms or protocols to manage warfarin dosing; using oral unit-dose products or prefilled syringes; ensuring ongoing monitoring of clinical and laboratory parameters for patients receiving antithrombotic therapy; educating staff on how to monitor for adverse effects of antithrombotic therapy; and implementing auditing tools to ensure compliance with facility policies.10,11,16 LTC providers should also make use of AMDA–Dedicated to Long Term Care Medicine’s recently released antithrombotic tool kit, which outlines policies and procedures and provides decision-support tools tailored to LTC practice.10
ACCP’s Assessment of Risks and Benefits
In 2012, the ACCP published guidelines regarding antithrombotic prophylaxis for patients with atrial fibrillation.5 These guidelines make recommendations for patients based on their stroke risk and on the presence of important comorbid conditions.5 When reviewing the available literature on antithrombotic prophylaxis for atrial fibrillation, these guidelines grade the strength of recommendations as strong (Grade 1) if benefits clearly outweigh risks, or weak (Grade 2) if the benefits and risks are similar. Within these categories, a recommendation is further rated based on the methodological quality of the studies assessed, from highest quality (A) to lowest quality (C).17 Most of the recommendations in the ACCP guidelines allow judgment and interpretation based on how patients perceive potentially positive and negative outcomes of therapy and on risk characteristics not captured by traditional risk models.
In the 2012 ACCP guidelines, the term oral anticoagulant refers to both warfarin and dabigatran, and of the two, dabigatran 150 mg twice daily was preferred to adjusted-dose warfarin (Grade 2B).5 Many of the atrial fibrillation recommendations in the guideline are Grade 2B; therefore, it is worth noting that this is a weak recommendation that is based on moderate-quality evidence and a close balance between desirable and undesirable effects.17
The 2012 ACCP guidelines note that all antithrombotic options for stroke prevention in the setting of atrial fibrillation are better than no treatment.5 In contrast to prior ACCP guidelines, they calculated no difference between current antithrombotic options with regard to all-cause mortality.5 Most patients in LTC will have CHADS2 scores ≥2 on the basis of age and the presence of at least one other risk factor. For patients with such CHADS2 scores, oral anticoagulation is preferred to no therapy (Grade 1A; NNT 67), to aspirin alone (Grade 1B; NNT 58), and to aspirin plus clopidogrel (Grade 1B; NNT 66).5 For those unwilling or unable to take oral anticoagulants, the guidelines recommend aspirin plus clopidogrel over aspirin alone (Grade 1B; NNT 38).5
(Continued on next page)
New Oral Anticoagulants
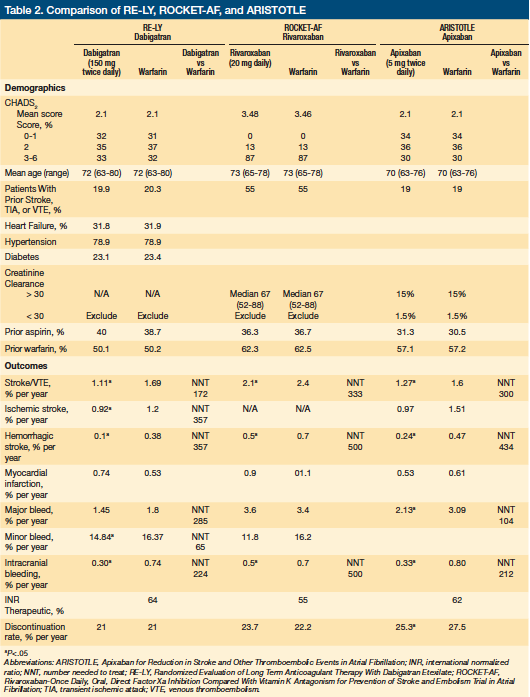
Three trials have compared warfarin to new anticoagulants for the prevention of stroke in nonvalvular atrial fibrillation: RE-LY, which compared warfarin to the direct thrombin inhibitor dabigatran18; ROCKET-AF, which compared warfarin to the factor Xa inhibitor rivaroxaban19; and ARISTOTLE, which compared warfarin to the factor Xa inhibitor apixaban20 (Table 2). These trials used similar (but not identical) inclusion and exclusion criteria; used different definitions for adverse events; and reported descriptive data about patients differently. The US Food and Drug Administration (FDA) has approved all three agents to prevent thromboembolism and stroke in patients with nonvalvular atrial fibrillation. Of these agents, apixaban is the most recently approved, having received approval on December 28, 2012.
RE-LY and Dabigatran
RE-LY enrolled 18,133 patients with atrial fibrillation from 951 centers in 44 countries. Patients were aged 75 years or older and had at least one of the following: previous transient ischemic attack (TIA) or stroke, left ventricular ejection fraction <40%, or New York Heart Association class II, III, or IV congestive heart failure.18 Younger patients (65 to 74 years) with atrial fibrillation were also enrolled, provided they had diabetes, hypertension, or coronary artery disease. The study excluded patients with prosthetic heart valves, hemodynamically relevant valvular heart disease, or a history of a disabling stroke within 6 months of study enrollment. Some of the exclusion criteria associated with increased risk of bleeding relevant to LTC patients included: an estimated creatinine clearance ≤30 mL/min; hemoglobin <10 g/dL; platelet count <100 x103/µL; major surgery in the previous month; history of intracranial or intraocular hemorrhage; gastrointestinal bleeding within the past 12 months; and malignancy or radiation therapy within 6 months of enrollment coupled with an estimated life expectancy <3 years.
During the study, 40% of patients took aspirin, which resulted in higher rates of bleeding than those observed with oral anticoagulants alone.21 Nearly 50% of patients had received long-term warfarin therapy before study enrollment, suggesting these patients were considered to be relatively low-risk for adverse effects by their treating physicians. International normalized ratios (INRs) were in the therapeutic range 64% of the time.
Most outcomes favored dabigatran 150 mg twice daily over warfarin with significantly lower rates of the primary outcome of stroke or systemic embolism (ARR 0.58%, NNT 172), hemorrhagic stroke (ARR 0.28%, NNT 357), life-threatening bleeding (ARR 0.35%, NNT 285), and intracranial bleeding (ARR 0.44%, NNT 227). Patients receiving dabigatran had a higher rate of myocardial infarction (ARI 0.21%, NNH 476) and gastrointestinal bleeding (ARI 204). No significant difference was found for all-cause mortality.
The RE-LY study compared warfarin to two doses of dabigatran, 150 mg or 110 mg twice daily. The FDA did not approve the 110-mg dose, but did approve a 75-mg dose twice daily for use in patients with a creatinine clearance between 15 mL/min and 30 mL/min. Clinicians should keep in mind that the RE-LY study specifically excluded patients with a creatinine clearance ≤30 mL/min and that no study exists comparing the 75-mg dose to warfarin for stroke prevention in atrial fibrillation.
ROCKET-AF and Rivaroxaban
ROCKET-AF enrolled 14,264 patients from 45 countries. All patients had atrial fibrillation and a moderate to high risk of stroke. Criteria for elevated stroke risk included a history of stroke, TIA, or systemic embolism, or at least two of the following: heart failure or ejection fraction ≤35%; hypertension; age ≥75 years; or diabetes.19 These risk factors ensured patients had a CHADS2 score ≥2. Clinical exclusion criteria included: hemodynamically significant mitral valve stenosis; a prosthetic heart valve; severe disabling stroke in the 3 months preceding enrollment; hemoglobin <10 g/dL; aspirin use of >100 mg daily; dual antiplatelet therapy; and a history of a major surgical procedure or trauma within 30 days of enrollment. Exclusion criteria related to bleeding risk included: creatinine clearance <30 mL/min; active internal bleeding; platelet count <90 x103/µL; gastrointestinal bleeding within the 6 months preceding enrollment; history of intracranial bleeding; known intracranial neoplasm; and intracranial arteriovenous malformation or aneurysm.
The relatively high prevalence of hypertension (90%), heart failure (63%), and diabetes (50%) compares favorably with the prevalence of these conditions in LTC patients. Additionally, 54% of study patients had a previous history of stroke, TIA, or systemic embolism. In addition, before enrollment, 36% were taking aspirin and 62% were taking warfarin. During the study, INR values were in the therapeutic range 55% of the time, a level of control below most research studies employing warfarin therapy. The investigators report that the efficacy of rivaroxaban was consistent across quartiles of duration of time in the therapeutic range; however, among centers with INR control in the lowest quartile, the differences between rivaroxaban and warfarin were no longer statistically significant. Most outcomes favored rivaroxaban over warfarin, with significantly lower rates of stroke or systemic embolism (ARR 0.3%, NNT 333), intracranial hemorrhage (ARR 0.2%, NNT 500), and critical bleeding (ARR 0.4%, NNT 250). No significant differences were found for rates of death or of any major bleeding or nonmajor bleeding.
ARISTOTLE and Apixaban
ARISTOTLE included 18,201 patients in 39 countries. Patients had atrial fibrillation at enrollment or had two documented episodes of atrial fibrillation in the 12 months preceding enrollment, along with at least one of the following stroke risk factors: prior stroke, TIA, or systemic embolism; age
≥75 years; symptomatic heart failure or ejection fraction <40%; diabetes; and hypertension.20 Exclusion criteria included a prosthetic heart valve; moderate or severe mitral stenosis; and increased bleeding risk, as evidenced by a previous intracranial hemorrhage, aspirin treatment >165 mg daily, dual antiplatelet therapy, life expectancy <1 year, renal insufficiency characterized by a serum creatinine >2.5 mg/dL or a calculated creatinine clearance <25 mL per minute, hemoglobin <9 g/dL, or a platelet count <100 x103/µL. Two doses of apixaban were administered: 5 mg twice daily for 95% of patients and 2.5 mg twice daily to 5% of patients. Patients receiving the lower dose met two or more of the following criteria: age 80 years or older, body weight <60 kg, or serum creatinine ≥1.5 mg/dL.
Of the study participants, 30% were 75 years or older; 19% had a previous stroke, TIA, or systemic embolism; 35% had heart failure; 25% had diabetes; 88% had hypertension; 17% had a history of clinically relevant or spontaneous bleeding; and 15% had a calculated creatinine clearance between 31 mL/min and 50 mL/min. Before enrollment, 57% were taking warfarin and 31% were taking aspirin. INR values were in the therapeutic range 62% of the time.
Most outcomes favored apixaban, with significantly lower rates of stroke or systemic embolism (ARR 0.33%, NNT 303), death from any cause (0.35%, NNT 238), hemorrhagic stroke (ARR 0.23%, NNT 435), major bleeding (0.96%, NNT 104), and intracranial bleeding (0.47%, NNT 212). There was no statistically significant difference in the rate of several outcomes: death from any cause, myocardial infarction, systemic embolism, or the composite of deep vein thrombosis or pulmonary embolism.
Of potential relevance to LTC patients, subset analyses suggested that older patients at higher risk showed the greatest benefit from taking apixaban versus warfarin.20 For example, the subgroup of patients younger than 65 years did not have a statistically significant decrease in rates of stroke or systemic embolism, whereas those older than 65 years did receive this benefit. Similarly, patients who had a CHADS2 score ≥3 showed a statistically significant reduction in stroke or systemic embolism; however, this same result was not observed in patients with a CHADS2 score of 1 or 2.
Convenience and Cost
The new oral anticoagulants offer convenience for both patients and physicians, as they do not require monitoring or dose adjustment, but this convenience comes at a cost. Warfarin is less expensive (approximately $4 per month) compared with dabigatran, rivaroxaban, and apixaban (approximately $260 per month).22 Even factoring in monitoring, warfarin is the more economical choice, costing about $55 per month.23
Cost-effectiveness estimates comparing dabigatran to warfarin suggest that the clinical benefits and convenience of the new oral anticoagulants come with an absolute increase in cost.23,24 Typically, a treatment is considered cost-effective if the cost of an additional quality-adjusted life year (QALY) is <$50,000. One model estimated that dabigatran produced 4.27 additional QALYs, compared with the 3.91 additional QALYs expected with warfarin use, at a cost of $25,000 per additional QALY.23 Another analysis estimated that an additional QALY derived from using dabigatran rather than warfarin would cost $86,000.24 As these data show, there is conflicting information regarding dabigatran’s cost effectiveness.
(Continued on next page)
Safety Considerations When Using the New Anticoagulants
The manufacturer of dabigatran advises caution if a patient is older than 75 years, has a creatinine clearance <30 mL/min, or is underweight, as these factors increase bleeding risk.22 In addition, clinicians should avoid prescribing concomitant medications that impact dabigatran’s absorption, as these may reduce its efficacy. Agents to avoid include high-dose pantoprozole (and perhaps other proton pump inhibitors) and inducers of P-glycoprotein, such as rifampin, carbamazepine, or St. John’s Wort.22,25 The risk of dabigatran-related bleeding is increased when excretion is reduced in advanced renal disease (creatinine clearance <30 mL/min), or if absorption is increased by P-glycoprotein inhibitors like verapamil, “azole” antifungals, quinidine, amiodarone, and dronedarone.22,25 Therefore, concomitant use of P-glycoprotein inhibitors should also be avoided.
Rivaroxaban is metabolized by CYP3A4 (50%) and eliminated by the kidneys (40%).26 For this reason, dose reduction is warranted if creatinine clearance is <50 mL/min.22 It is best to avoid medications that are strong inhibitors of both P-glycoprotein and CYP3A4, such as “azole” antifungals, clarithromycin, conivaptan, and ritonavir.22,26,27 P-glycoprotein inducers (eg, rifampin, carbamazepine, or St. John’s Wort) should also be avoided because they may decrease rivaroxaban’s effectiveness.22,26,27
Apixaban is metabolized primarily by CYP3A4 and is a substrate for P-glycoprotein. Therefore, it is contraindicated for patients receiving medications that are strong inhibitors of both CYP3A4 and P-glycoprotein, such as “azole” antifungals, clarithromycin, conivaptan, and ritonavir, as these medications may increase the risk of bleeding events.22,28,29 Medications that are strong inducers of both CYP3A4 and P-glycoprotein, like carbamazepine, rifampin, phenobarbital, and St. John’s Wort, should also be avoided because they may decrease the effectiveness of apixaban.22,28,29 In addition, because renal impairment increases the concentration of apixaban, it is contraindicated if a patient’s creatinine clearance is <15 mL/min, and it should be used cautiously if his or her creatinine clearance is between 16 mL/min and 29 mL/min.22,28,29 As with all oral anticoagulants, including warfarin, coadministration with aspirin or nonsteroidal anti-inflammatory drugs increases the risk of bleeding complications, and adding clopidogrel to apixaban doubles the risk of bleeding.22
Conclusion
The new oral anticoagulants may offer important clinical benefits, notably decreased intracranial hemorrhages and decreased rates of stroke in carefully selected patients. They are also convenient for providers and patients, but this convenience comes at a significant cost increase over warfarin. However, the clinical trials of these new agents either did not include, or specifically excluded, the types of patients and comorbid conditions that are so common among LTC patients. Product prescribing information may not expressly prohibit use for these conditions and circumstances, but many of the study exclusion criteria targeted increased bleeding risk. Thus, if a patient has one or more study exclusion criteria, the increased risk of bleeding may eliminate the expected safety benefit of the newer agents. For such patients, clinicians may choose either to avoid the new oral agents or to counsel the patient about the potential increase in bleeding risk and monitor them appropriately. If a new agent is used in a high-risk situation (eg, use of dual antiplatelet therapy), increased clinical and laboratory monitoring for bleeding would be prudent. Finally, the new agents should only be used for FDA-approved conditions. For example, use of these new oral anticoagulants is currently being investigated for patients with valvular heart disease. Until studies show benefits, patients should not receive these agents for this condition. In addition, a recent study of dabigatran for mechanical valvular prophylaxis was stopped early due to safety concerns, highlighting the need for careful clinical trials before clinicians prescribe these agents for conditions not approved by the FDA, particularly for patients residing in LTC settings.
References
1. Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008:133(6 Suppl):546S-592S.
2. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370-2375.
3. English KM, Channer KS. Managing atrial fibrillation in elderly people. BMJ. 1999;318(7191):1088-1089.
4.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983-988.
5. You JJ , Singer, DE, Hoyard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e531S-575S.
6. Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med. 1996;349:1019-1026.
7. Man-Son-Hing M, Laupacis A. Anticoagulant-related bleeding in older persons with atrial fibrillation: physicians' fears often unfounded. Arch Intern Med. 2003;163(13):1580-1586.
8. Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151(5):297-305.
9. Miles RW. Fallacious reasoning and complexity as root causes of clinical inertia.
J Am Med Dir Assoc. 2007;8(6):349-354.
10. Smucker WD, Riddle A. American Medical Directors Association. Antithrombotic Therapy in the Long-term Care Setting Tool Kit Series. Columbia, MD: 2012.
11. Smucker WD, Kamel H. American Medical Directors Association. Atrial Fibrillation Long-term Care Tool Kit Series. Columbia, MD: 2011.
12. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radfor MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864-2870.
13. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093-1100.
14. Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57(2):173-180.
15. Maclean W, Mulla S, Akl EA, et al. Patient values and preferences in decision making for antithrombotic therapy: a systematic review: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e1S-e23S.
16. The Joint Commission. National Patient Safety Goals Effective January 1, 2012. Long-term Care Accreditation Program. www.jointcommission.org/assets/1/6/NPSG_Chapter_Jan2012_LTC.pdf. Accessed on December 20, 2012.
17. Guyatt GH, Norris SL, Schulman S, et al. Methodology for the development of antithrombotic therapy and prevention of thrombosis guidelines: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):53S-70S.
18. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151.
19. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891.
20. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992.
21. Moia M, Mannucci PM. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(27):2672; author reply 2674-2675.
22. PL Detail-Document. Comparison of Oral Antithrombotics. Prescriber’s Letter. 2011.
23. Kamel H, Johnston SC, Easton JD, Kim AS. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in patients with atrial fibrillation and prior stroke or transient ischemic attack. Stroke. 2012;43(3):881-883.
24. Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation. 2011;123(22):2562–2570.
25. Horn JR, Hansten PD. Dabigatran: A New Oral Anticoagulant. Pharmacy Times. www.pharmacytimes.com/publications/issue/2010/December2010/DrugInteractions-1210. Published December 13, 2010. Accessed December 20, 2012.
26. Horn JR, Hansten PD. Rivaroxaban: A New Oral Anticoagulant. Pharmacy Times. www.pharmacytimes.com/publications/issue/2012/February2012/Rivaroxaban-A-New-Oral-Anticoagulant. Published February 20, 2012. Accessed December 20, 2012.
27. PL Detail-Document. Oral Anticoagulants: Maximizing Safety. Pharmacist’s Letter. 2012.
28. Nutescu E. Apixaban: a novel oral inhibitor of factor Xa. Am J Health Syst Pharm. 2012;69(13):1113-1126.
29. New drugs: Apixaban [newsletter]. Aust Prescr. 2011;34:153-159.
Disclosures:
The author reports no relevant financial relationships.
Address correspondence to:
William D. Smucker, MD
Summa Health System
55 Arch St, Suite 3A
Akron, OH 44304
billsmucker@neo.rr.com










