Antimicrobial Stewardship in Long-Term Care Facilities: An Opportunity for Intervention
Abstract: Antimicrobial stewardship programs (ASPs) in long-term care (LTC) facilities are needed to prevent development of resistance and potential adverse effects associated with inappropriate antimicrobial use. LTC facilities serve as major environmental reservoirs for transmission of various multidrug-resistant organisms. The Centers for Medicare & Medicaid Services released a ruling that requires LTC facilities to have infection control measures in place, including an ASP, to aid in preventing transmission of these resistant pathogens. The Centers for Disease Control and Prevention recommends initiatives, such as developing geographically-focused or facility-specific antibiograms, staff education, implementation of antimicrobial therapy algorithms for common infections, and automatic stop-order policies, as ways to combat bacterial resistance. Potential challenges to the development and implementation of ASPs in LTC facilities include inadequate resources, opposition from providers, and lack of administrative support. Despite these challenges, establishing an ASP in the LTC setting is needed to protect both residents and the greater public by decreasing the development of resistance pathogens and promoting optimal antimicrobial use. This review provides insight into successful antimicrobial stewardship practices in LTC facilities.
Key words: antimicrobial stewardship, long-term care facility, long-term care, geriatric
The development of antimicrobial resistance remains one of the major global public health threats today.1 Antimicrobial stewardship programs (ASPs) are important to help curb this problem because they foster appropriate antimicrobial use. In acute care facilities, ASPs have been well described, but there is a dearth of data regarding ASPs in long-term care (LTC) facilities in both acute and chronic settings. Patients in LTC facilities, due to their advanced age and preexisting comorbid conditions, are at an increased risk of complications secondary to an infection.1 LTC facilities serve as major environmental reservoirs for transmission of various multidrug-resistant organisms. Oftentimes, residents undergo prolonged courses of antibiotics, which is a risk factor for acquiring colonization with multidrug-resistant organisms.2,3 Despite these known risks, approximately 10% of residents receive antibiotics, half of which are inappropriate or unnecessary.4-6
Inappropriate use of antibiotics can lead to antimicrobial resistance, adverse effects, Clostridium difficile infection, and unnecessary costs.7 These factors have led to average estimated expenditures between $38 million to $137 million on antibiotics per year in the LTC setting.8 Furthermore, the growing older adult population in the United States calls attention to the need for developing ASPs in LTC facilities. According to the US Census Bureau, the US population is projected to increase from 318.7 million in 2014 to 416.8 million in 2060, with an increasing distribution of older adults in the population over the coming decades.9 By 2029, 1 in 5 US residents will be aged 65 or older, which is a substantial increase from 1 in 7 in 2014.9
There are multiple challenges to performing adequate stewardship in LTC facilities. A lack of resources, such as availability of onsite providers or accessibility of microbiologic and diagnostic testing, are common. The culture within a particular facility may also promote less-than-desirable antimicrobial prescribing practices.10 Lack of knowledge, disinterest in facility initiatives, and lack of consideration of input from other health care workers have also been cited as perceived barriers to the implementation of successful ASP initiatives.10 The LTC patient population contains a high prevalence of comorbidities, including cognitive impairment and incontinence. This can make clinical assessment difficult and lead to uncertain diagnoses that may increase antimicrobial usage.
Despite these challenges, the importance of developing ASPs should not be discounted because their potential to improve patient care and outcomes has been extensively proven.11-14 As a part of its national action plan to prevent health care associated infections, the Department of Health and Human Services (HHS) included ASPs as an important component of LTC facility infection control efforts.15 Acknowledging the challenges, HHS also urged further development of ASP practices in LTC facilities to facilitate understanding of what should constitute best practice. Since then, the Centers for Medicare & Medicaid Services (CMS) have included ASP efforts among a myriad of new requirements for LTC facilities.16 The need for antibiotic usage monitoring and protocols was highlighted, as was the role of pharmacists in assisting with stewardship.
The purpose of this article is to provide a comprehensive systematic review of successful antimicrobial stewardship practices in LTC facilities.
Methods
We searched PubMed, EBSCO, and EMBASE electronic databases (up to August 12, 2017) for publications written in the English language describing interventions and outcomes of ASPs in LTC facilities. Search terms included “nursing home,” “antimicrobial stewardship,” “long-term care facilities,” “antibiotic use,” and “resistance.” References from relevant articles were reviewed to assess for inclusion. Articles chosen for inclusion in the review quantitatively assessed the impact of an intervention aimed to improve antimicrobial usage in either a nursing home (NH), skilled nursing facility (SNF), or LTC facility. In addition, surveys that assessed the current antimicrobial stewardship practices in either an NH, SNF, or LTC facility were included. The intervention performed in each study and associated outcomes were summarized. Of the 48 studies identified, 23 studies were included and 25 were excluded. Studies taking place in long-term acute care hospitals, due to the vast differences in practice settings and available resources when compared with LTC facilities, were excluded from our review.
Results
Eighteen studies were found describing stewardship interventions aimed at changing prescribing practices, and 5 surveys describing stewardship practices were found (Supplementary Table 1). A variety of study designs, ranging from mail-in survey questionnaires to cluster randomized controlled trials, were used.
Five studies specifically assessed the impact of prescribing for urinary tract infections (UTIs), and 3 additional studies evaluated prescribing for pneumonia. Interventions relied heavily on providing education, and many had multidisciplinary involvement.
Pneumonia-Focused Outcomes
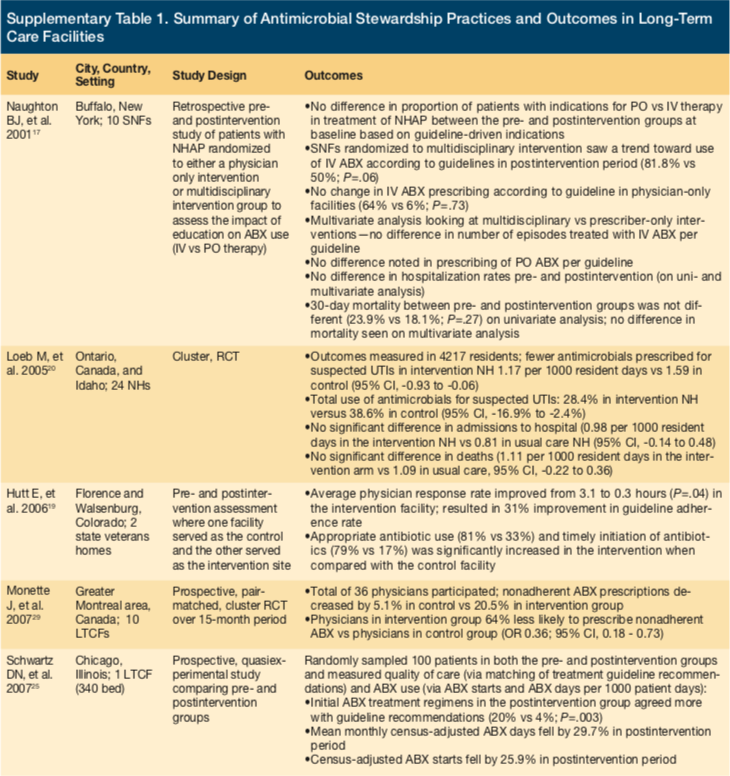
Naughton et al conducted a retrospective chart review in New York examining the impact of prescribing for the treatment of NH-acquired pneumonia (NHAP) using a guideline-directed, multidisciplinary approach compared with a provider-only method.17 Ten facilities were randomized, and comparisons were made using preintervention and postintervention data. Prescribers had opportunity to assist with the development of guidelines, which were based on preimplementation data. Education about the guidelines was also provided. In facilities randomized to a multidisciplinary approach, nurses were educated and encouraged to actively seek solutions to implementation barriers. The education did not affect adherence to the new guideline on univariate analysis, either preimplementation vs postimplementation or a multidisciplinary vs provider-only approach. However, multivariate analysis of postimplementation data demonstrated an increase in NHAP cases treated with intravenous (IV) antibiotics according to guidelines. There were no differences in rates of hospitalization or 30-day mortality.
Linnebur et al investigated the effects of implementing NHAP treatment guidelines in a quasiexperimental study at 16 NHs in Colorado, Kansas, and Missouri.18 Rather than using institution-specific data to create guidelines, national evidence-based guidelines were implemented by a multidisciplinary team of physicians, pharmacists, and nurses at each site. Prescribers and nurses were educated about content, which included antimicrobial prescribing and diagnostic criteria. Additionally, an appointed site-specific liaison nurse was responsible for continuing education among staff, helping solve barriers to implementation, and encouraging use of the guidelines. While these efforts increased timely administration of antimicrobials, no significant differences were seen in overall prescribing of guideline-recommended antimicrobials, adherence to duration of therapy recommendations, or mortality. Those with chest x-ray results suggesting pneumonia were more likely to receive guideline-recommended antimicrobials.
A study by Hutt et al had a similar design to Naughton et al.19 Investigators worked with nursing staff, pharmacists, and administration to develop and implement procedures and guidelines for improving treatment of NHAP in 2 NHs. Similar education was provided to nurses and prescribers and resulted in improvements in care. Patients were more likely to receive appropriate and timely antibiotics. Physician response also significantly improved, but no differences were seen in rates of hospitalization or mortality.
UTI-Focused Outcomes
Loeb et al compared outcomes of an educational intervention aimed at improving UTI treatment in a cluster randomized controlled trial.20 Twenty-four NHs from Ontario, Canada, and Idaho were included; 12 served as intervention, and 12 as control. Nurses and prescribers were educated on the UTI diagnostic and treatment algorithm via small group sessions, videos, written materials, and investigator visits. As a result, intervention sites decreased antimicrobial use for suspected UTIs, but hospitalization and mortality rates remained unchanged.
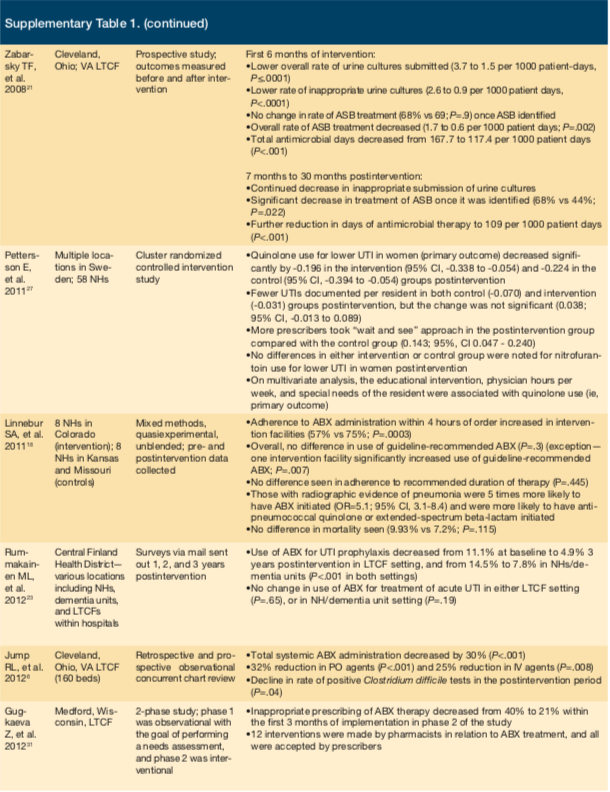
Zabarsky et al prospectively investigated outcomes before and after implementation of a UTI diagnostic and treatment guideline at an LTC facility in Ohio.21 Verbal and written instruction was used to educate nurses and prescribers about the lack of benefit in treating asymptomatic bacteriuria, appropriate indications for obtaining urine cultures, and adverse effects of unnecessary antimicrobials. The study showed not only a reduction in total antimicrobial days postintervention, but also a change in the practice of urine culture obtainment. There were lower rates of inappropriate and total urine cultures submitted for analysis, leading to an overall decreased rate of asymptomatic bacteriuria treatment. Results were sustained up to 30 months.21
Kassett et al compared outcomes related to antimicrobial use and UTI diagnosis before and after implementation of an ASP at a 472-bed LTC facility and a 255-bed geriatric specialty hospital.22 This study used a part-time clinical pharmacist and 2 part-time family medicine physicians to implement a program focused on 4 stewardship strategies: (1) development of ASP guidelines and policies; (2) information technology tools (eg, default stop dates); (3) multidisciplinary education; and (4) prospective audit with feedback. In the LTC facility, no difference was demonstrated in overall antibiotic use. However, there was a significant difference in both the prescribed and actual duration of therapy following ASP implementation. Rates and proportions of ciprofloxacin use were also significantly lower in the postimplementation period.22
Rummakainen et al attempted to show a sustained reduction in antimicrobial use for UTIs at 25 primary care hospitals and 39 NHs throughout Finland.23 Prior to guideline implementation, a nurse, geriatrician, and infectious diseases specialist visited each site to administer questionnaires assessing baseline practices, from which guidelines for UTI treatment were constructed. Facilities received surveys by mail at 1 year, 2 years, and 3 years postintervention to assess long-term efficacy. While there was no change over the years in the use of antimicrobials for treatment of UTI, researchers found a sustained decrease in the use of antimicrobials for UTI prophylaxis.
Doernberg et al assessed the effect of a weekly prospective audit with feedback on antibiotic use for the treatment of UTIs in 3 community LTC facilities.24 Opportunities for interventions were reviewed by an ASP team comprised of an infectious diseases pharmacist and infectious diseases physician. After initiation of the ASP intervention, antibiotic UTI prescriptions immediately decreased 26%, with a 6% reduction that continued throughout the 6-month intervention period. This study also assessed reductions in overall antibiotic prescriptions for all indications and found a 25% immediate decrease after the start of the intervention and a 5% reduction throughout the intervention period (95% CI: -8% to -2%). While the authors concluded that weekly ASP interventions reduced antibiotic use, many opportunities were left unaddressed due to resource limitations. Of note, antibiotics were not indicated in 85% of reviewed prescriptions. Compared with the acute care setting, developing relationships with prescribers in the LTC setting is significantly more difficult because much of the medical care occurs remotely. In addition, LTC providers felt pressured by nursing staff and resident families to send laboratory tests and cultures for nonspecific, nondiagnostic changes, thus resulting in excessive unnecessary treatment.
Studies Aiming to Decrease Antibiotic Use
The remaining studies focused on antimicrobial use on a broader scale rather than a particular disease state. Many of these studies incorporated multidisciplinary involvement.25-27
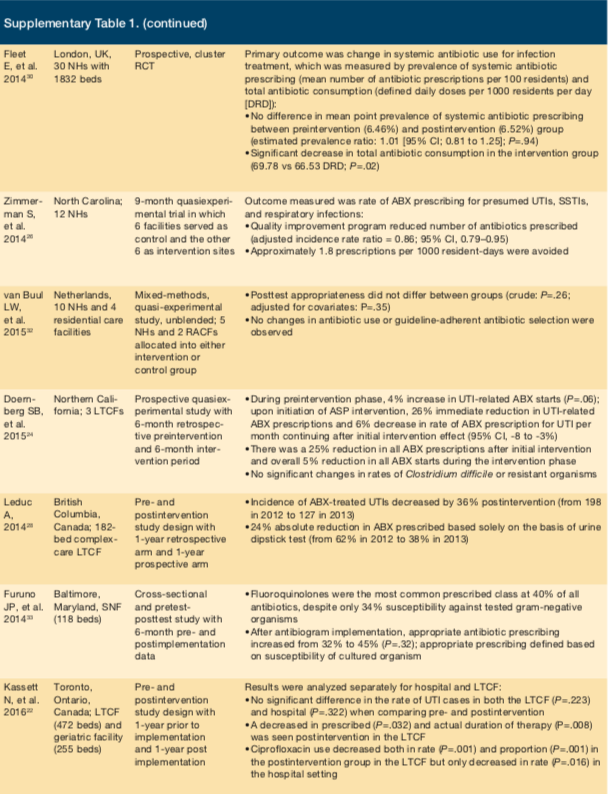
Schwartz et al conducted a study at an LTC facility in Chicago, assessing the effects of education sessions targeting prescribers and nurses.25 The sessions addressed resistance rates within the institution and provided diagnostic and treatment algorithms for UTI, respiratory tract infections (RTIs), central venous catheter infections, diarrhea, infected pressure ulcers, and diabetic foot infections. The sessions also targeted scenarios where prescribing antimicrobials was inappropriate (eg, asymptomatic bacteriuria). These efforts resulted in a nearly 30% decrease in antimicrobial use and a 26% reduction in initiation of antimicrobials. Prescribed antimicrobials were also more likely to be in accordance with national guidelines. A study by Zimmerman et al, which assessed a very similar intervention on a larger scale in 12 NHs in North Carolina (6 control and 6 intervention)26 and a cluster, randomized control trial, study by Pettersson et al, involving 58 NH in Sweden27 both documented a decrease in antimicrobial usage.
Another study by Leduc embraced an entirely nurse-driven approach to reducing antibiotic use targeting
asymptomatic bacteriuria.28 Nurses were educated with a UTI clinical pathway as well as a proprietary UTI Self-Learning Package designed to help them assess, manage, and monitor residents with suspected UTIs. Nurses contacted physicians with relevant clinical information to aid the provider in accurately diagnosing true UTIs and minimizing treatment of asymptomatic bacteriuria. Overall incidence of antibiotic-treated UTIs decreased 36% postintervention (from 198 in 2012 to 127 in 2013). The LTC facility experienced a 24% absolute reduction in inappropriately prescribed antibiotics solely on the basis of a urine dipstick test without a clinical assessment (from 62% in 2012 to 38% in 2013). By incorporating other etiologic causes of nonspecific UTI symptoms within the clinical pathway, nurses were able to identify patients with dehydration who responded to fluids alone but otherwise may have received antibiotics for a presumptive UTI.
In 2 studies, investigators decided to forego a multidisciplinary approach.29,30 A prospective, cluster, pair-matched, randomized, controlled trial by Monette et al included 8 LTC facilities in Montreal, Canada, to determine the impact of educational handouts on antimicrobial prescribing patterns.29 Educational handouts along with individual physician prescribing practices from a previous time period were mailed to prescribers. Pharmacists were involved in the development of educational materials based on antimicrobial availability within each facility. Handouts included recommendations for empiric antimicrobials, dosing and frequency of antimicrobials, and suggested duration of therapy for treatment of UTI, lower RTIs, skin and soft tissue infections, and septicemia of unknown origin. Despite the relatively passive nature of the intervention, a substantial increase in appropriateness of prescribed antibiotics was achieved.
While the study by Monette et al29 targeted only prescribers, a study by Fleet et al focused on nurses to drive changes in antimicrobial use.30 This prospective, cluster, randomized controlled trial was conducted at 30 NHs in London. Nurses were instructed to complete a Resident Antimicrobial Management Plan for each patient prescribed an antimicrobial (Table 1). The first part was filled out prior to the first dose of an antibiotic and included details of the physician assessment and diagnosis, appropriateness of antibiotic choice, and documentation of antibiotic administration. The second part was filled out 48 hours to 72 hours after initiation of an antibiotic to document patient progress, the intended duration of therapy, and outcomes. Overall, this strategy had a modest effect. The number of prescriptions for antimicrobials per 100 residents did not change, but there was a small yet significant decrease in defined daily doses per 1000 residents per day.

Two studies focused on using an ASP team to actively review antibiotic orders and make recommendations to prescribers.6,31 Jump et al conducted a study at a Cleveland LTC facility that successfully impacted patient outcomes.6 The facility created an infectious-diseases consult service consisting of a physician and nurse practitioner. This service saw patients in the facility weekly and was available for consultation. Through this team, a 30% decrease in overall antibiotic use occurred; of note, use of fluoroquinolone and antipseudomonal agents was significantly decreased postimplementation. Additionally, a decrease in C difficile infection occurred. Similarly, a single-site study in a Wisconsin LTC facility tasked pharmacists with reviewing and suggesting antimicrobial therapy to providers. These interventions, too, successfully resulted in reduced antimicrobial use.31
Only 1 study failed to find any benefit from an intervention.32 This study was conducted in 10 NHs in the Netherlands and used a participatory action research approach. The multidisciplinary approach sought engagement of stakeholders and involved several cycles of data collection, data analysis, identification of improvement opportunities, and implementation of improvement measures. The goal was to create improvement measures that addressed barriers and issues at each institution, resulting in a uniquely suited program for each facility. Unfortunately, this method did not have any measurable effect on antimicrobial prescribing.
Antibiograms have improved antibiotic prescribing practices in acute care, but their impact in SNFs is largely unknown. A study by Furuno et al created an antibiogram using a 6-month period of culture data followed by educational inservices at 3 SNFs (118 beds) in Maryland.33 Improvements in empiric antibiotic prescribing were evaluated after the intervention. Authors defined appropriate antibiotic prescribing as providing antibiotic therapy active against the resultant cultured organism. Preintervention data identified fluoroquinolones (40%) as the most commonly prescribed class of antibiotics despite only 34% susceptibility against tested gram-negative organisms. After antibiogram implementation, appropriate antibiotic prescribing increased from 32% to 45% (P=.32). Statistical significance was not achieved due to the lack of positive culture results and low specimen collection frequency, study authors noted. While this was a study limitation, the findings reinforce the necessity of stewardship tools such as an antibiogram to help guide therapeutic decisions.
Surveys of Antimicrobial Stewardship Practices
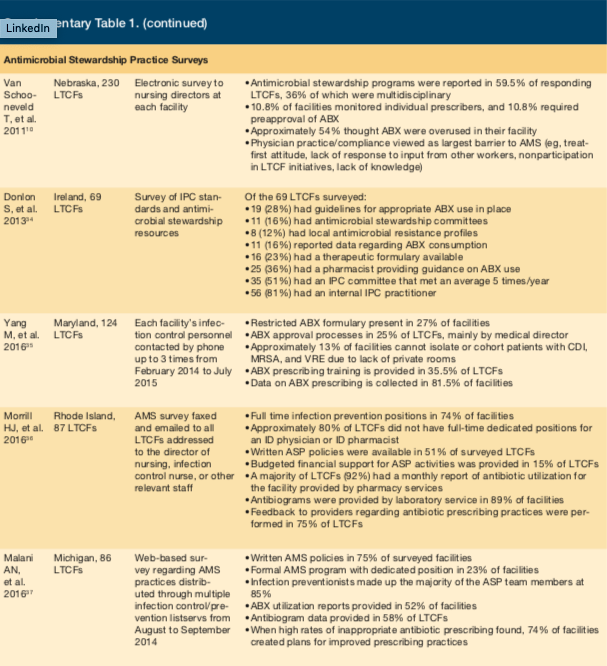
Five surveys that focused on implementation of antimicrobial stewardship practices in LTC facilities were identified.10,34 Van Schooneveld et al performed a systematic survey of all LTC facilities in Nebraska and determined that 59.5% of respondents had ASPs in place. This was defined by implementation of programs to ensure appropriate antibiotic use or stewardship champions responsible for monitoring antibiotics.10 Although a majority of LTC facilities had ASPs, stewardship practices varied, and only 36% involved multiple health care professionals. The most common practice included monitoring resistant pathogens, mainly methicillin-resistant Staphylococcus aureus (97.3%) and measuring antibiotic use (81.1%). Prescriber-directed education (8.1%) and preapproval of antibiotics (10.8%) were rarely performed. A majority of respondents (54.1%) believed antibiotics were overused, and almost all (89.2%) believed that ASPs were beneficial in LTC. The primary perceived barrier to ASP implementation was physician practice or compliance (69.2%).10 Other barriers identified were patient or family expectations (15.4%) as well as system (7.7%) and communication (7.7%) issues.10
Donlon et al found similar practices when surveying Irish LTC facilities, although fewer had ASPs in place (16%).34 A common practice involved using pharmacists to provide guidance on antibiotic use (36%). Similar to Van Schooneveld et al, prescriber-directed education (7%) was rarely noted as a stewardship practice. Both surveys found fewer than 10% of LTC facilities had prescriber-directed training, fewer than 25% had an antimicrobial formulary, and fewer than 30% had antibiotic protocols or guidelines. Sites with a dedicated physician were more likely to have staff training and guidelines.10 Results of these surveys indicate significant gaps in antimicrobial stewardship practices, which may be due to a lack of resources and perceived barriers (eg, physician compliance and system issues).10,34
A survey of infection control personnel at 124 LTC facilities was performed by Yang et al throughout Maryland.35 Data on antibiotic prescribing practices was found in 81.5% of surveyed facilities. While this finding is encouraging, opportunities for significant improvement with other measures and practices were identified. Restricted antibiotic formularies were present in 27% of facilities, while an antibiotic approval process was used in 25%. Training for antibiotic prescribing practices was provided in 35.5% of LTC facilities. Due to a lack of private rooms, approximately 13% of LTC facilities were unable to isolate (individually or by cohorts) patients with methicillin-resistant S aureus, vancomycin-resistant Enterococci, and C difficile. While 90.3% of all surveyed LTC facilities had central line protocols, 6% of facilities did not admit a patient with a central line.
Morrill et al surveyed 87 LTC facilities in Rhode Island via email and fax about their antimicrobial stewardship practices.36 Full-time infection preventionists were used in 74% of facilities, while the majority of LTC facilities (80%) lacked full-time dedicated positions for a physician or pharmacist trained in infectious diseases. The study suggested infection preventionists had a significant role in antimicrobial stewardship within these LTC facilities, but more practitioners trained in infectious diseases with expertise in direct patient care were needed. Approximately half of the surveyed facilities had written ASP policies, although only 15% provided financial support for ASP-related activities. Survey questions regarding ASP monitoring practices received a positive response, with a majority of facilities (92%) providing access to pharmacy-run monthly antibiotic utilization reports. In 89% of LTC facilities, laboratory services provided antibiograms or other reports detailing the facility’s antibiotic resistance. Feedback to providers on antibiotic prescribing practices was provided in 75% of facilities.
Malani et al surveyed 86 LTC facilities throughout Michigan on ASP practices by providing links to a web-based survey on infection control listservs.37 This study identified high use of infection preventionists for ASP-related interventions (85%). Additionally, written ASP policies were available in 75% of surveyed facilities, but only 23% of LTC facilities provided dedicated positions for formal ASP-related roles. Antibiotic utilization reports were used in approximately 50% of facilities and 58% of facilities provided antibiogram data. When high rates of inappropriate antibiotic prescribing were identified, 74% of facilities said they created plans for improving prescribing practices. Continued training, education, and support for antimicrobial stewardship practices were all considered crucial to mitigating further development of antibiotic resistance in the LTC setting.
Discussion
LTC facilities admit medically complex patients often transferred from acute care hospitals with high rates of antibiotic use. As such, LTC facilities have been implicated in outbreaks of multidrug-resistant organisms. At any given time, 7% to 10% of LTC residents may be taking an antimicrobial, and 50% to 70% will receive at least one course annually.4,38,39 A similar systematic review reported that 47% to 79% of NH residents received systemic antimicrobial agents each year, and antimicrobials were prescribed for 77% to 88% of all infectious episodes.40 Reviews have consistently demonstrated a very high proportion of inappropriate antimicrobial use in LTC facilities.41
Determining whether a resident needs an antimicrobial is often difficult because typical signs and symptoms of infection (eg, fever, rising leukocytosis) are frequently absent in older adults. Additionally, much of the medical care occurs remotely and, due to limited availability of clinicians, few residents see a prescriber on a daily basis. Developing systems and resources that optimize antimicrobial use in LTC will improve the quality of antibiotic therapy delivered, decrease development of resistance strains and the incidence of C difficile infections, and help manage antibiotic drug costs.
CMS published a final rule October 4, 2016, that mandates establishment of an infection prevention and control program in LTC facilities. The rule, titled Medicare and Medicaid Programs; Reform of Requirements for Long-Term Care Facilities, requires an expansion of infection control interventions, including the development and implementation of antimicrobial stewardship activities in each facility.16 Elements will be implemented in a 3-stage process. Phase 1 requirements order the establishment of an infection prevention and control program with a system for preventing, identifying, reporting, investigating, and controlling infections and communicable disease. Efforts must consider staff, visitors, volunteers, and others performing contracted services to the facility and include: (1) written standards; (2) policies and procedures that address when and how isolation will be used for a resident; (3) a system for recording incidents identified under the facility’s infection prevention and control program; and (4) corrective actions taken by the facility and an ASP. An ASP is part of phase 2 and should have been implemented by November 28, 2017. The ASP must include “antibiotic use protocols and antibiotic monitoring.”
Key elements common to most successful ASPs identified by the Centers for Disease Control and Prevention. and in this review, include but are not limited to developing geographically focused or facility-specific antibiograms, education development, and implementation of diagnosis and empiric antimicrobial therapy algorithms for common infections in LTC facilities (eg, UTIs, cellulitis, upper RTIs, etc.). Developing automatic stop-order policies, sometime referred to as antibiotic timeouts, is also important. Antimicrobial orders should have a defined dose, duration (stop date), and indication. Clinicians should obtain cultures before initiating therapy; once culture data are reported, clinicians should reassess the choice of therapy, considering a move from broad-spectrum to bacteria-specific drugs and developing algorithms to transition from IV to oral therapy after the resident is stabilized and able to tolerate an oral diet. A list of restricted antimicrobials requiring active review and ongoing surveillance to assure appropriate use should be developed. Also, monitoring of the development of C difficile infections, the incidence of resistant bacteria, and prescribing patterns should occur.
Challenges to the development and implementation of an ASP in LTC facilities have been well documented.42 Lack of organizational awareness of benefits of an ASP may contribute to inadequate resources, whether it is a lack of funding, staff, expertise, or technological support. Additional barriers within a facility can include opposition from providers or competing initiatives that do not foster the goals of an ASP. The manner in which care is provided in LTC facilities adds additional challenges, as prescribing clinicians are frequently not in the facility every day and information for the prescriber’s clinical assessment is often garnered via fax or phone without a physical exam. Daneman et al found that fewer than 44% of residents received a physician visit within 1 day of antimicrobial therapy initiation.39 It seems intuitive that for the development and implementation of successful ASPs in LTC, we will need to be creative in generating systems to deploy essential infectious diseases practitioners (eg, physicians, nurse practitioners, and pharmacists) and clinically meaningful data in the most cost-effective manner possible.
Conclusion
Despite the challenges and obstacles to developing an ASP in LTC, it is incumbent to establish best practices to protect both residents and the greater public by helping decrease development of multidrug-resistant organisms and promoting safe, effective, and appropriate antimicrobial use.
Supplementary materials for this article can be found within the PDF at the end of the article.
References
1. Juthani-Mehta M, Quagliarello VJ. Infectious diseases in the nursing home setting: challenges and opportunities for clinical investigation. Clin Infect Dis. 2010;51(8):931-936.
2. Venkatachalam I, Yang HL, Fisher D, et al. Multidrug-resistant gram-negative bloodstream infections among residents of long-term care facilities. Infect Control Hosp Epidemiol. 2014;35(5):519-526.
3. Lim CJ, Cheng AC, Kennon J, et al. Prevalence of multidrug-resistant organisms and risk factors for carriage in long-term care facilities: a nested case-control study. J Antimicrob Chemother. 2014;69(7):1972-1980.
4. Pakyz AL, Dwyer LL. Prevalence of antimicrobial use among United States nursing home residents: results from a national survey. Infect Control Hosp Epidemiol. 2010;31(6):661-662.
5. Nicolle LE, Bentley DW, Garibaldi R, Neuhaus EG, Smith PW. Antimicrobial use in long-term-care facilities. SHEA Long-Term-Care Committee. Infect Control Hosp Epidemiol. 2000;21(8):537-545.
6. Jump RL, Olds DM, Seifi N, et al. Effective antimicrobial stewardship in a long-term care facility through an infectious disease consultation service: keeping a LID on antibiotic use. Infect Control Hosp Epidemiol. 2012;33(12):1185-1192.
7. Chopra T, Goldstein EJ. Clostridium difficile infection in long-term care facilities: a call to action for antimicrobial stewardship. Clin Infect Dis. 2015;60(suppl 2):S72-76.
8. Cohen CC, Jeong Choi Y, Stone PW. Costs of infection prevention practices in long-term care settings: a systematic review. Nurs Econ. 2016;34(1):16-24.
9. Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. US Census Bureau website. Published March 2015. Accessed November 28, 2018.
10. Van Schooneveld T, Miller H, Sayles H, Watkins K, Smith PW. Survey of antimicrobial stewardship practices in Nebraska long-term care facilities. Infect Control Hosp Epidemiol. 2011;32(7):732-734.
11. Carling P, Fung T, Killion A, Terrin N, Barza M. Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect Control Hosp Epidemiol. 2003;24(9):699-706.
12. Sanders J, Pallotta A, Bauer S, et al. Antimicrobial stewardship program to reduce antiretroviral medication errors in hospitalized patients with human immunodeficiency virus infection. Infect Control Hosp Epidemiol. 2014;35(3):272-277.
13. Liew YX, Lee W, Tay D, et al. Prospective audit and feedback in antimicrobial stewardship: is there value in early reviewing within 48 h of antibiotic prescription? Int J Antimicrob Agents. 2015;45(2):168-173.
14. Avdic E, Cushinotto LA, Hughes AH, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis. 2012;54(11):1581-1587.
15. US Department of Health and Human Services. Chapter 8: Long-Term Care Facilities. In: National Action Plan to Prevent Health Care-Associated Infections: Road Map to Elimination. April 2013.
16. Medicare and Medicaid programs; reform of requirements for long-term care facilities. Fed Regist. 2016;81(192):68688-68872.
17. Naughton BJ, Mylotte JM, Ramadan F, Karuza J, Priore RL. Antibiotic use, hospital admissions, and mortality before and after implementing guidelines for nursing home-acquired pneumonia. J Am Geriatr Soc. 2001;49(8):1020-1024.
18. Linnebur SA, Fish DN, Ruscin JM, et al. Impact of a multidisciplinary intervention on antibiotic use for nursing home-acquired pneumonia. Am J Geriatr Pharmacother. 2011;9(6):442-450.e1.
19. Hutt E, Ruscin JM, Corbett K, et al. A multifaceted intervention to implement guidelines improved treatment of nursing home-acquired pneumonia in a state veterans home. J Am Geriatr Soc. 2006;54(11):1694-1700.
20. Loeb M, Brazil K, Lohfeld L, et al. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. BMJ. 2005;331(7518):669.
21. Zabarsky TF, Sethi AK, Donskey CJ. Sustained reduction in inappropriate treatment of asymptomatic bacteriuria in a long-term care facility through an educational intervention. Am J Infect Control. 2008;36(7):476-480.
22. Kassett N, Sham R, Aleong R, Yang D, Kirzner M, Craft A. Impact of antimicrobial stewardship on physician practice in a geriatric facility. Can J Hosp Pharm. 2016;69(6):460-465.
23. Rummukainen ML, Jakobsson A, Matsinen M, et al. Reduction in inappropriate prevention of urinary tract infections in long-term care facilities. Am J Infect Control. 2012;40(8):711-714.
24. Doernberg SB, Dudas V, Trivedi KK. Implementation of an antimicrobial stewardship program targeting residents with urinary tract infections in three community long-term care facilities: a quasi-experimental study using time-series analysis. Antimicrob Resist Infect Control. 2015;4:54.
25. Schwartz DN, Abiad H, DeMarais PL, et al. An educational intervention to improve antimicrobial use in a hospital-based long-term care facility. J Am Geriatr Soc. 2007;55(8):1236-1242.
26. Zimmerman S, Sloane PD, Bertrand R, et al. Successfully reducing antibiotic prescribing in nursing homes. J Am Geriatr Soc. 2014;62(5):907-912.
27. Pettersson E, Vernby A, Mölstad S, Lundborg CS. Can a multifaceted educational intervention targeting both nurses and physicians change the prescribing of antibiotics to nursing home residents? A cluster randomized controlled trial. J Antimicrob Chemother. 2011;66(11):2659-2666.
28. Leduc A. Reducing the treatment of asymptomatic bacteriuria in seniors in a long-term care facility. Can Nurse. 2014;110(7):25-30.
29. Monette J, Miller MA, Monette M, et al. Effect of an educational intervention on optimizing antibiotic prescribing in long-term care facilities. J Am Geriatr Soc. 2007;55(8):1231-1235.
30. Fleet E, Gopal Rao G, Patel B, et al. Impact of implementation of a novel antimicrobial stewardship tool on antibiotic use in nursing homes: a prospective cluster randomized control pilot study. J Antimicrob Chemother. 2014;69(8):2265-2273.
31. Gugkaeva, Z, Franson M. Pharmacist-led model of antibiotic stewardship in a long-term care facility. Annals of Long-Term Care: Clinical Care and Aging. 2012;
20(10):22-26.
32. van Buul LW, van der Steen JT, Achterberg WP, et al. Effect of tailored antibiotic stewardship programmes on the appropriateness of antibiotic prescribing in nursing homes. J Antimicrob Chemother. 2015;70(7):2153-2162.
33. Furuno JP, Comer AC, Johnson JK, et al. Using antibiograms to improve antibiotic prescribing in skilled nursing facilities. Infect Control Hosp Epidemiol. 2014;35(suppl 3):S56-61.
34. Donlon S, Roche F, Byrne H, Dowling S, Cotter M, Fitzpatrick F. A national survey of infection control and antimicrobial stewardship structures in Irish long-term care facilities. Am J Infect Control. 2013;41(6):554-557.
35. Yang M, Vleck K, Bellantoni M, Sood G. Telephone survey of infection-control and antibiotic stewardship practices in long-term care facilities in Maryland. J Am Med Dir Assoc. 2016;17(6):491-494.
36. Morrill HJ, Mermel LA, Baier RR, et al. Antimicrobial stewardship in Rhode Island long-term care facilities: current standings and future opportunities. Infect Control Hosp Epidemiol. 2016;37(8):979-982.
37. Malani AN, Brennan BM, Collins CD, Finks J, Pogue JM, Kaye KS. Antimicrobial stewardship practices in Michigan long-term care facilities. Infect Control Hosp Epidemiol. 2016;37(2):236-237.
38. Loeb M, Simor AE, Landry L, et al. Antibiotic use in Ontario facilities that provide chronic care. J Gen Intern Med. 2001;16(6):376-383.
39. Daneman N, Gruneir A, Newman A, et al. Antibiotic use in long-term care facilities. J Antimicrob Chemother. 2011;66(12):2856-2863.
40. van Buul LW, van der Steen JT, Veenhuizen RB, et al. Antibiotic use and resistance in long term care facilities. J Am Med Dir Assoc. 2012;13(6):568.e1-13.
41. Smith PW, Bennett G, Bradley S, et al. SHEA/APIC guideline: infection prevention and control in the long-term care facility. Infect Control Hosp Epidemiol. 2008;
29(9):785-814.
42. Chopra T, Rivard CM. Effective antibiotic stewardship programs at long-term care facilities: a silver lining in the post-antibiotic era. Annals of Long-Term Care: Clinical Care and Aging. 2015;23(3):18-23.










