Anorexia of Aging
As people age, they become less active and normal physiologic changes cause a shift in body composition, with an increase in proportion of body fat and a decrease in lean muscle mass and extracellular fluid mass.1 The body typically responds to the decrease in energy needs with a desire for fewer calories. This predisposes older adults to anorexia of aging, a syndrome associated with unplanned weight loss and protein-energy malnutrition (PEM).2,3 The Centers for Medicare & Medicaid Services (CMS) defines significant unplanned or undesired weight loss as a 5% decline in body weight over 1 month, 7.5% over 3 months, and 10% over 6 months (Table 1).4 Various physiologic, pathologic, psychologic, and sociologic factors (eg, depression, loss of social networks, chronic illness, medications) increase the risk of anorexia of aging and resultant malnutrition.

Although it is widely recognized that maintaining a well-balanced and nutritious diet plays a vital role in maintaining good health during the aging process, malnutrition is becoming increasingly prevalent among the elderly population. Despite its prevalence, undernutrition—defined as protein-energy deficiency that is reversed solely by the administration of nutrients5—often goes undiagnosed in the elderly. Undernutrition is associated with an increased risk of mortality and morbidity, including frailty, pressure ulcers, impaired wound healing, dehydration, and functional decline.4 Failure to assess long-term care (LTC) residents for nutritional status and to document efforts to correct avoidable cases of undernutrition can result in deficiency citations from CMS for Tag F325.4
In addition to discussing the causes of anorexia of aging, this article reviews three validated instruments that are useful for assessing the nutritional status of geriatric patients. Interventions are also examined, with conclusions drawn from the literature about their effectiveness in addressing this syndrome. Understanding why some elderly people eat less as they get older, to the point where they experience unhealthy weight loss and PEM, will help healthcare providers learn how to assess for risk factors associated with anorexia of aging.
Prevalence of Protein-Energy Malnutrition
Elderly individuals often consume smaller meals, eat more slowly, drink less, and take fewer snacks between meals, all of which contribute to a reduction in calorie intake.6,7 One study reported a 25% decrease in daily calorie consumption from 40 to 70 years of age.8 Another study comparing differences in calorie consumption between individuals aged 25 years and 70 years found that older men’s daily caloric intake dropped by 1000 to 1200 kcal and older women took in 600 to 800 kcal less per day.5
A decline in activity and energy expenditure over time means fewer calories are needed to maintain body weight—one study9 estimated that an adult’s daily energy use declines 100 kcal each decade of life—and this helps balance the decrease in calories consumed. Any factor that further affects eating habits or increases energy requirements, however, can upset this balance, leading to significant weight loss or an unsafe decline in body mass index (BMI) and undernutrition.
In developed countries, approximately 85% of LTC residents, between 23% and 62% of hospitalized elderly patients, and 15% of community-dwelling older adults suffer from malnutrition.10,11 A recent meta-analysis of geriatric conditions commissioned by the US Preventive Services Task Force (USPSTF) noted that the lack of a universal standard for diagnosing malnutrition in the elderly makes it difficult to assess its prevalence.12 The USPSTF study determined that older African Americans were significantly more likely than white people to experience unintended weight loss; and while the authors found no difference in the risk of unintended weight loss between Hispanics and non-Hispanics, older Hispanic women were significantly more likely to have poor nutritional scores than older women who were not Hispanic (30% vs 17%, respectively).12
In the community and LTC settings, many age-related physiologic changes,medical conditions, medication use, mental health issues, and social factors contribute to an increased risk of anorexia of aging (Table 2). It has been reported that more than two-thirds of LTC residents aged ≥65 years voluntarily restrict their diet and reduce calorie consumption.7 Some factors may be modifiable, whereas others are not. In those cases, additional measures are needed to halt and hopefully reverse unintended weight loss and undernutrition.

Continued on next page
Medical Causes of Anorexia of Aging
Various physical ailments—some of which are debilitating—and their respective treatments can depress appetite or cause problems that interfere with eating. Conditions such as chronic obstructive pulmonary disease (COPD), Parkinson’s disease, and arthritis cause inflammation and impair mobility.13 Older adults are also prone to gastrointestinal disorders or malabsorption problems,11 which can contribute to decreased food intake and unintentional weight loss, as can constipation and fecal impaction.4 Cognitive impairment can also lead to weight loss13; individuals affected by Alzheimer’s disease or dementia, for example, may experience loss of appetite or forget to eat.14 The aging body also undergoes many physiologic and pathologic changes that can inhibit desire for and enjoyment of food.
Sensory Perception of Food
Taste, smell, sight, and texture are essential components of food enjoyment.15 People whose senses of smell and taste are diminished tend to experience fewer food cravings and demonstrate less positive involvement with food to the point where they may even question the purpose of eating. Doty and colleagues16 found that >60% of study participants between 65 and 80 years of age and >80% of participants ≥80 years had impaired taste and smell compared with taste and smell sensitivity among participants ≤50 years.
Dysfunction in taste and smell among the aged is usually attributable to chemosensory loss, but it can also result from chronic disease, medication use, and PEM.17 More than 250 prescription medications alter taste,11 whereas other drugs affect smell. Changes in how the taste and smell of foods are perceived typically contribute to poor dietary choices, decreased appetite, and low nutrient intake.10,11
Older adults experience “an increase in the taste threshold, difficulty in recognizing taste mixtures, an increased perception of irritating tastes, and a decreased number of supertasters.”3 Many studies suggest that our threshold for detecting specific tastes (eg, sweet, salty, bitter) increases with age and that certain medications can reduce the number of functioning taste buds and decrease taste sensitivity.18 One study reported that elderly individuals who took a moderate number of medications were less able to detect certain tastes at average threshold levels.17 Studies comparing taste perception between younger and older adults found that the elderly participants rated a broad range of tastes as less intense. People with a diminished sense of smell also demonstrate less interest in food and eat less.6
Studies have shown that using additives to imbue foods with more flavor helps compensate for age-related chemosensory losses.11 One study found that adding natural flavors to regular foods increased food intake among older hospitalized patients by 13% to 26%.7 Serving foods with pleasant aromas, such as freshly baked bread, may also help stimulate appetite. In addition, residents may find food more appealing if it is familiar, recognizable (not mushed together), seasoned, and served at the correct temperature.5,7,15
Many LTC residents have illnesses that warrant restrictions on amounts of fat, sugar, and sodium, and they find their meals less satisfying.5 The American Dietetic Association (ADA) notes that “a strict, unappealing therapeutic diet is not beneficial unless it is actually consumed.”5 In Tag F325, CMS encourages diet liberalization and recommends facilities consider temporarily lifting dietary restrictions for residents who are losing weight, undernourished, or at risk because they are not eating properly.4 CMS cautions nursing homes to consider the resident’s preferences in meal planning and recognize his or her right to decline recommended dietary restrictions.
Delayed Stomach Emptying (Gastroparesis)
Older adults who have suppressed appetites due to earlier and prolonged satiation are more likely to eat smaller and fewer meals, which can lead to insufficient calorie intake and malnutrition. Delayed stomach emptying, or gastroparesis, can lead to early satiation and complaints of fullness early on during mealtime. Other symptoms of gastroparesis include postprandial vomiting, nausea, abdominal pain, weight loss, and nutritional deficiencies.19 A study by Di Francesco and associates8 observed elderly individuals for 4 hours after they consumed an 800-kcal meal and found that gastric emptying was delayed by more than 2 hours, satiety lasted longer, and hunger was suppressed throughout the observation period.
With age, there are changes in the gastrointestinal sensory function, which can lead to premature feelings of fullness in older adults.10 According to Morley,3 aging is associated with damage to the receptive relaxation of the gastric fundus, which results in more rapid antral filling, gastric distension, and prolonged satiation.Delayed stomach emptying appears to be a normal consequence of aging, involving “reduced sensitivity to gastrointestinal distension”10 and slower emptying rates.7 Distension of the stomach is a key indication to end the meal, however, because of impaired receptive relaxation, which results in rapid antral filling, older adults perceive satiation before they have consumed enough calories to satisfy their nutritional needs.3
The hormone cholecystokinin (CCK), which is secreted by the proximal bowel in response to satiation and helps mediate gastric emptying, may also play a role. Studies suggest that sensitivity to the effects of CCK increase with age6 and that older adults have higher levels of CCK.10 The increased level of circulating CCK, combined with increased sensitivity, may slow antral emptying.6
Gastroparesis is also associated with certain illnesses, such as type 2 diabetes, which is estimated to account for one-third of cases.20 One study reported that 88% of Parkinson’s patients had delayed gastric emptying of solids and 38% had delayed gastric emptying of liquids.21 Other causes include damage to the vagus nerve during esophageal or stomach surgery; scleroderma; pancreatitis; imbalances in potassium, calcium, or magnesium; certain medications, especially narcotics and anticholinergic agents; and thyroid disease.20
A gastric emptying study can be used to determine whether a patient is experiencing delayed stomach emptying.19,20 Negative results may indicate the need to look for physical obstruction, such as a tumor. The diet for a resident with gastroparesis should be low in fat and fiber, which delay the transit of food through the stomach, and contain soft foods in small portions.20 Promotility drugs and enteral nutrition are other options for consideration. When possible, the use of pain-relieving narcotics and anticholinergics in individuals with gastroparesis should be avoided.19,20
Other Physiologic Factors
In addition to CCK, several other hormones may contribute to unintentional weight loss. Leptin, a peptide hormone produced in adipose tissue, helps maintain energy balance. The amount of circulating leptin is directly related to the size of fat stores.6 Di Francesco and colleagues8 noted that “low leptin levels signal loss of body fat and a need for energy intake, while high leptin levels testify [to] the presence of adequate body fat and no need for further food intake.” Research suggests that older adults have higher levels of leptin than younger adults.10,22 As the proportion of body fat increases with age, leptin levels may also rise, signaling the body to eat less.
Insulin, a satiety hormone, is another regulator of glucose metabolism. Insulin increases appetite by enhancing leptin signaling to the hypothalamus and inhibiting secretion of ghrelin, a hormone that stimulates appetite.10 Some researchers have found no relationship between insulin levels and the anorexia of aging, however,concluding that higher insulin levels are a byproduct of insulin resistance and insulin resistance is a response to an increase in body adiposity, rather than an effect of aging.6 Further research is needed to clarify what role, if any, insulin plays in unintended weight loss.
Continued on next page
Chewing and Swallowing Problems
Mouth conditions, poor dentition, or ill-fitting dentures can make chewing difficult, causing patients to limit food selection, thus interfering with energy intake.11 In the United States, 23% of individuals aged 65 to 75 years and 36% of adults ≥75 years have severe periodontal disease, and 30% of adults ≥65 years are toothless.23 Many LTC residents also have problems with chewing and swallowing (dysphagia) due to stroke, pain, lethargy, dry mouth, confusion, upper gastrointestinal disease,4 and neurologic disease. Between 50% and 75% of nursing home residents are thought to have dysphagia.24 The causes of swallowing and chewing issues should be investigated and appropriate interventions taken. It may be something as simple as positioning the patient properly during meals or could be more involved, such as treating an underlying medical condition (eg, gastroesophageal reflux disease, dental problems).4
Hypermetabolic Conditions
Some illnesses raise the patient’s resting metabolic rate, increasing energy and protein requirements. Cancer is strongly associated with anorexia-cachexia, a syndrome of progressive loss of adipose tissue and muscle mass triggered by a tumor-related increase in the patient’s basal metabolic rate.25 Significant weight loss often precedes diagnosis of a tumor,25 and cancer should be included in the differential diagnosis for anorexia of aging.
Other conditions associated with a hypermetabolic state, such as wound recovery, infections (eg, pneumonia), fevers, advanced COPD, and hyperthyroidism, increase energy and protein requirements. Practitioners should watch for signs of undernutrition in these patients even before evidence of weight loss.4 It may be prudent to encourage residents with postsurgical wounds or chronic ulcers or who are at risk of wounds (ie, pressure ulcers) to eat as much protein as they can tolerate.4
Medication Use
Many elderly adults routinely take prescription medications for multiple medical conditions, such as hypertension, pain, high cholesterol levels, and breathing problems. Drugs used to treat these and other conditions can cause dry mouth, metallic taste, nausea, vomiting, constipation, and diarrhea—adverse effects that typically inhibit the desire to eat. Adverse effects of chemotherapy, particularly nausea and oral mucositis, can also decrease energy intake. Some drugs, such as digoxin and metformin, cause malabsorption of one or more nutrients.26 As noted previously, anticholinergics and narcotics can slow digestion and increase the risk of unintended weight loss. CMS notes that it may be necessary to “change, stop, or reduce the doses” of drugs associated with an increased risk of weight loss to stabilize weight for a resident who is not eating well.4
Sociologic and Psychologic Factors
Although residents in LTC facilities face challenges different from those of community-dwelling elders—many of whom experience declining income after retirement and may struggle to shop for food or prepare meals—older adults in both settings often lose motivation to eat.11,13 People generally associate mealtimes with company, conversation, and a pleasant atmosphere, which are not always available to LTC residents, who frequently experience the loss of their social networks and may have little control over their dining situation.
Isolation and Depression
The loss of a spouse and friends or changes in the daily routine that accompany retirement and institutionalization can contribute to social isolation and, in some cases, feelings of depression and loneliness that dampen the desire to eat.3 Van Staveren and colleagues13 reported that older adults eat larger meals when eating in a group versus eating alone. A study of elderly adults living in inner city areas showed having a visitor during mealtimes reduced an elderly person’s risk of developing dysphoria and improved food intake.27 Caregivers can cultivate the enjoyment and social aspects of eating by encouraging older adults to eat with someone.13
Depression can also be pathologic. Between 30% and 40% of patients with Parkinson’s disease develop depression.28 Higher rates of depression have also been reported for individuals with Alzheimer’s disease, vascular dementia, cardiovascular disease, type 2 diabetes mellitus, arthritis, cancer, and stroke, although it is not clear whether these cases of depression are connected with the underlying disease process.29,30 Unlike younger adults with depression, who tend to increase food consumption, studies indicate that older adults eat less when they are depressed.18 Depression screening may be needed, followed by appropriate interventions to relieve depression.5
Loss of Independence in LTC
Some residents may refuse to eat in response to limited food choices and stipulated mealtimes in the nursing home. Residents may also eat less when caretakers fail to provide sufficient attention to the resident and his or her needs during mealtime.13 According to van Staveren and associates,13 33% of adults ≥65 years of age need assistance with activities of daily living,and staff may need to make eating easier for these patients by cutting food into bite-sized pieces, opening packets, removing lids, buttering bread,5 and providing assistance with utensils.
CMS is increasingly encouraging person-centered care at LTC facilities,4 contributing to a change in the culture of care at nursing homes that promotes residents’ independence, especially when it comes to dining.5 Some facilities are offering family-style mealtimes; more freedom to choose when, where, and what to eat; buffet-style dining; restaurant-style menus; five meals instead of three; and social functions with snacks (Table 3).5
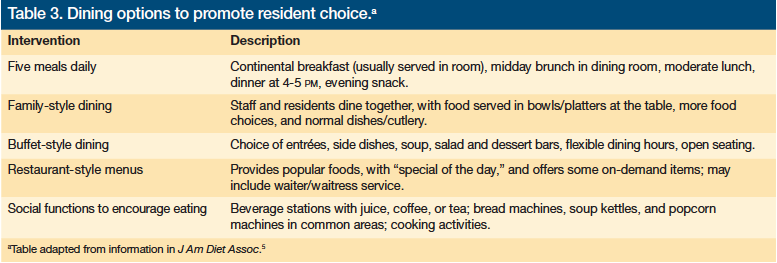
A randomized controlled study found that Dutch nursing home residents who were given their meals family-style for 6 months significantly increased energy intake by a mean of 115 kcal, compared with a meal decline of 100 kcal in the control group.31 The family-style diners maintained body weight, physical and psychosocial function, and quality of life, all of which declined significantly in the control group.
Continued on next page
Assessing Nutritional Status in LTC Residents
The ADA defines nutritional assessment as “a systematic process of obtaining, verifying, and interpreting data in order to make decisions about the nature and cause of nutrition-related problems.”4 CMS requires LTC facilities to assess every resident’s nutritional status as part of the Resident Assessment Instrument (RAI), and develop a care plan for individuals at risk of undernutrition. Several other more detailed assessment tools are available and should be used
to supplement the RAI when appropriate.4 Despite the availability of these assessment tools, Volkert and colleagues32 found significant inconsistencies between the presence of malnutrition in patients admitted to a geriatric hospital ward and its diagnosis by physicians and nurses.
A complete nutritional assessment often requires an interdisciplinary team that includes caregivers in a position to observe the resident during mealtimes, the resident or his or her representative, and healthcare practitioners.4 Other experts who might need to be part of the team are a registered dietician (if on staff),5 a speech pathologist to investigate swallowing issues, a psychologist if depression is suspected, and a pharmacist.4,33
Signs of Undernutrition
Measuring height and weight is the least invasive way to assess nutritional status and allows BMI to be calculated. In the LTC setting, it is important to weigh residents at admission to establish a baseline weight, then weekly for 4 weeks and monthly thereafter.4 Studies differ on the ideal BMI for adults aged ≥65 years, but most agree that a BMI of <18.5 kg/m2, which is considered underweight in adults,34 is inadequate for assessing the nutritional status of elderly adults. Volkert and colleagues32 identified a BMI of <22 kg/m2 as indicative of malnutrition in adults aged ≥65 years.32 Other studies35 have found higher mortality rates in elderly individuals with a BMI <25 kg/m2, which suggests that more research is needed to determine the usefulness of BMI in detecting malnutrition in the elderly.
Other indicators of nutritional status include overall appearance; condition of the hair, nails, and skin; oral health; and behavior, such as degree of responsiveness or lethargy.4 Laboratory values, such as serum albumin levels, may point to possible malnutrition but should be followed with a more thorough assessment.4
Screening Instruments Validated for Geriatric Use
The Mini Nutritional Assessment (MNA) is a validated, reliable instrument specifically designed for use in older adults and should be included in a comprehensive geriatric assessment.36 Using the MNA, Kaiser and colleagues37 found a high prevalence of malnutrition in a select group of elderly patients and, due to the tool’s geriatric focus, recommended it form the “the basis for nutritional evaluation in older people.”The MNA has also been shown to help predict mortality risk.10
The approximately 15-minute test contains 18 items, including height, weight, mobility, lifestyle, circumference of the arm and calf, weight loss during the previous 3 months, eating and drinking habits, medication use, presence of pressure ulcers, ability to feed oneself, and the patient’s perception of his or her health and nutritional status.38 A maximum of 14 points are possible, with a score of 12 to 14 points indicating “normal nutritional status,” 8 to 11 points indicating “at risk of malnutrition,” and 0 to 7 points indicating a patient who is malnourished. The MNA short form was recently released, which consists of six questions and is available at no cost for the iPod and iPhone.
Another reliable assessment tool for malnutrition risk is the Sadness, Cholesterol, Albumin, Loss of Weight, Eating, and Shopping (SCALES) test.10 SCALES was designed for outpatient screening and combines a subjective assessment with laboratory results to determine malnutrition risk. Variables considered include a score of >15 out of 30 on the Geriatric Depression Scale, cholesterol concentration <4.14 mmol/L, an albumin concentration of <4 g/L, a loss of 2 lb of body weight in 1 month or 5 lb in 6 months, difficulty feeding oneself, and the ability to buy and prepare meals.42 A score of 3 or higher is an indication of malnutrition.43 Thomas and associates42 performed a study in a subacute care setting and eliminated shopping because it did not apply to the population.42 They discovered SCALES, without the shopping assessment, is a useful tool in determining malnutrition risk. By eliminating the shopping question, the SCALES assessment would be an appropriate tool for patients in LTC.
The Geriatric Nutritional Risk Index (GNRI) is used to evaluate risk of nutrition-related problems. Like the MNA, the GNRI contains anthropometric components such as height, weight, triceps skinfold, and arm circumference, but it adds nutritional markers, including albumin, prealbumin, and total lymphocytes.39 A GNRI score <92 indicates high risk, 92 to 98 is moderate risk, and >98 is no risk. In comparing the GNRI with the MNA in 241 institutionalized elderly residents, Cereda and colleagues39 found poor agreement between the two assessments regarding nutritional
status and concluded that the GNRI was better than the MNA at predicting nutrition-related complications. The researchers noted that since the GNRI was newer than the MNA and the MNA had several studies attesting to its validity, combining the two would be the best option for predicting nutrition-related outcomes.39
These tests can be performed by the resident’s primary care provider (physician or nurse practitioner), or by a registered dietician. It is important that whoever is performing the assessmment is familiar with the tool, so they do not come to an incorrect conclusion.
Care Plan
Whatever method of assessment is used, once a resident has been identified as being at risk for undernutrition or as malnourished, a care plan must be developed. In addition to some of the previously discussed interventions, practitioners will want to weigh the need, benefits, and risks of medical treatment, nutritional supplementation, or even feeding tube use. Many older individuals are set in their ways, and it can be difficult to change their daily routines. Practitioners should consider the individual’s current eating habits and preferences when developing a care plan.
Once interventions have been implemented for patients with unplanned weight loss, frequent monitoring of their effectiveness is important. Patients should be weighed weekly until their weight stabilizes or improves. The care plan should be updated as the patient’s status changes, when new interventions are tried, or to reflect new goals.4
Appetite Stimulants
Current evidence does not support using appetite-stimulating medications to treat unintentional weight loss in older adults, although CMS notes their use may be appropriate in limited circumstances.4 Trials involving dronabinol, megestrol acetate, and recombinant growth hormone have reported serious treatment-related adverse effects, and these agents are not recommended for regular clinical use, especially in frail elders.40
Nutritional Supplementation
Nutritional supplementation has been shown to improve caloric intake among individuals with anorexia of aging, reducing their risk of malnutrition.10 Payette and associates41 found that providing nutrient-dense, protein-energy liquid supplements to frail elderly people who were undernourished and losing weight significantly improved their nutritional status and arrested or reversed weight loss. Another study reported weight gain and a 34% reduction in mortality risk with oral nutritional supplementation.40
Timing of nutritional supplementation is important, because it may cause the patient to eat less during mealtime. Studies indicate that consuming nutritional supplements between meals, rather than with meals, is associated with greater improvement in caloric intake. It may also help to administer nutritional supplements at the time medications are given.4
Feeding Tubes
Feeding tubes may be an option for some patients, such as those with dementia or nearing the end of life, but the resident’s preferences must be considered and respected. CMS notes that tube feeding has not been found to extend or improve quality of life in patients with dementia.4
Conclusion
Anorexia of aging and PEM are major concerns among the aging population and especially in LTC. Unintentional weight loss in an elderly nursing home resident must be taken seriously because it increases the risk of morbidities, such as pressure ulcers, diminished cognitive and physical function, and falls; raises the likelihood of hospitalization; and is associated with an elevated risk of mortality.
Although many of the age-related physiologic, pathologic, sociologic, and psychologic changes associated with PEM are inevitable, PEM is usually preventable and should not be considered part of the normal aging process. Avoiding poor—and often costly—outcomes associated with undernutrition depends on identifying LTC residents at risk, conducting a proper nutritional assessment, and promptly taking any necessary preventive or corrective measures. Facilities would also benefit from adopting mealtime practices that enhance enjoyment of eating for all residents. While it is important to educate residents on good nutrition, these conversations should deemphasize food restrictions and seek to encourage healthy, sufficient food intake.
The author reports no relevant financial relationships.
Ms. Champion is a registered nurse and a master’s-level student, University of Pennsylvania, Philadelphia, PA.
References
1. Noel M, Reedy M. Nutrition and aging. Prim Care. 2005;32(3):659-669.
2. Horwitz B, Blanton C, McDonald R. Physiologic determinants of the anorexia of aging: insights from animal studies. Annu Rev Nutr. 2002;22:417-438.
3. Morley JE. Pathophysiology of anorexia. Clin Geriatr Med. 2002;18(4):661-673,v.
4. US Centers for Medicare & Medicaid Services. State Operations Manual. Appendix PP - Guidance to Surveyors for Long Term Care Facilities. Section 483.25(i). Rev. 70. https://www.cms.gov/manuals/downloads/som107ap_pp_guidelines_ltcf.pdf. Revised January 7, 2011. Accessed September 15, 2011.
5. Dorner B, Friedrich EK, Posthauer ME. Practice paper of the American Dietetic Association: individualized nutrition approaches for older adults in health care communities. J Am Diet Assoc. 2010;110(10):1554-1563.
6. MacIntosh C, Morley JE, Chapman IM. The anorexia of aging. Nutrition. 2000;16(10):983-995.
7. Nieuwenhuizen W, Weenen H, Rigby P, et al. Older adults and patients in need of nutritional support: review of current treatment options and factors influencing nutritional intake. Clin Nutr. 2010;29(2):160-169.
8. Di Francesco V, Fantin F, Omizzolo F, et al. The anorexia of aging. Dig Dis. 2007;25(2):129-137.
9. Wells JL, Dumbrell AC. Nutrition and aging: assessment and treatment of compromised nutritional status in frail elderly patients. Clin Interv Aging. 2006;1(1):67-79.
10. Ahmed T, Haboubi N. Assessment and management of nutrition in older people and its importance to health. Clin Interv Aging. 2010;5:207-216.
11. Donini L, Savina C, Cannella C. Eating habits and appetite control in the elderly: the anorexia of aging. Int Psychogeriatr. 2003;15(1):73-87.
12. Kane RL, Talley KMC, Shamilyan T, Pacala JT. Common syndromes in older adults related to primary and secondary prevention. Evidence Report/Technology Assessment; vol 87. Rockville, MD: Agency for Healthcare Research and Quality, 2011. www.uspreventiveservicestaskforce.org/uspstf11/es87.pdf. Published July 2011. Accessed September 28, 2011.
13. van Staveren WA, de Graaf C, de Groot LC. Regulation of appetite in frail persons. Clin Geriatr Med. 2002;18(4):675-684.
14. Johansson L, Sidenvall B, Malmberg B, Christensson L. Who will become malnourished? A prospective study of factors associated with malnutrition in older persons living at home. J Nutr Health Aging. 2009;13(10):855-860.
15. Morley JE. Anorexia of aging: physiologic and pathologic. Am J Clin Nutr. 1997;66(4):760-773.
16. Doty RL, Shaman P, Applebaum S, et al. Smell identification ability: changes with age. Science. 1984;226(4681):1441-1443.
17. Schiffman S, Graham B. Taste and smell perception affect appetite and immunity in the elderly. Eur J Clin Nutr. 2000;54(suppl 3):S54-S63.
18. Roberts S, Rosenberg I. Nutrition and aging: changes in the regulation of energy metabolism with aging. Physiol Rev. 2006;86(2):651-667.
19. National Institute of Diabetes and Digestive and Kidney Diseases. Gastroparesis. Bethesda, MD: National Institutes of Health; 2007. NIH publication 07-4348. https://digestive.niddk.nih.gov/ddiseases/pubs/gastroparesis/Gastroparesis.pdf. Published July 2007. Accessed September 15, 2011.
20. Parkman HP, Camilleri M, Farrugia G, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS Meeting. Neurogastroenterol Motil. 2010;22(2):113-133.
21. Goetze O, Nikodem AB, Wiezcorek J, et al. Predictors of gastric emptying in parkinson’s disease. Neurogastroenterol Motil. 2006;18(5):369-375.
22. Zamboni M, Zoico E, Fantin E, et al. Relation between leptin and the metabolic syndrome in elderly women. J Gerontol A Biol Sci Med. 2004;59(4):396-400.
23. National Institute of Dental and Craniofacial Research. A plan to eliminate craniofacial, oral, and dental health disparities. www.nidr.nih.gov/research/healthdisp/hdplan.pdf. Revised February 2002. Accessed August 16, 2011.
24. Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124(1):328-336.
25. National Cancer Institute. Nutrition in cancer care (PDQ). https://www.cancer.gov/cancertopics/pdq/supportivecare/nutrition/HealthProfessional/page1. Revised July 2011. Accessed September 15, 2011.
26. Sampson G. Weight loss and malnutrition in the elderly: the shared role of GPs and APDs. Aust Fam Physician. 2009;38(7):507-510.
27. Suda Y, Marske CE, Flaherty JH, et al. Examining the effect of intervention to problems of the elderly living in an inner city area: a pilot project. J Nutr Health Aging. 2001;5(2):118-123.
28. Frisina PG, Haroutunian V, Libow LS. The neuropathological basis for depression in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(2):144-148.
29. Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851-858.
30. Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54(3):
216-226.
31. Nijs KA, de Graaf C, Kok FJ, van Staveren WA. Effect of family style mealtimes on quality of life, physical performance, and body weight of nursing home residents: cluster randomised controlled trial. BMJ. 2006;332(7551):1180-1184.
32. Volkert D, Saeglitz C, Gueldenzoph H, et al. Undiagnosed malnutrition and nutrition-related problems in geriatric patients. J Nutr. 2010;14(5):387-392.
33. Huffman GB. Evaluating and treating unintentional weight loss in the elderly. Am Fam Physician. 2002;65(4):640-651.
34. US Centers for Disease Control and Prevention. Healthy weight: it’s not a diet, it’s a lifestyle! https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/eng
lish_bmi_calculator/results_underweight.html?pounds=100&inches=64. 2011. Updated May 4, 2011. Accessed February 11, 2011.
35. Kvamme JM, Holmen J, Wilsgaard T, et al. Body mass index and mortality in elderly men and women: the Tromso and HUNT studies [published online ahead of print Feburary 14, 2011]. J Epidemiol Community Health. doi:10.11.1136/jech.2010.123232. Accessed September 28, 2011.
36. Guigoz Y. The Mini Nutritional Assessment (MNA) review of the literature—What does it tell us? J Nutr Health Aging. 2006;10(6):466-485.
37. Kaiser M, Bauer J, Ramsch C, et al; Mini Nutritional Assessment International Group. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58(9):1734-1738. doi:10.1111/j.1532-5415.2010.03016.x.
38. Izawa S, Kuzuya M, Okada K, et al. The nutritional status of frail elderly with care needs according to the mini-nutritional assessment. Clin Nutr. 2006;25(6):962-967.
39. Cereda E, Pusani C, Limonta D, Vanotti A. The ability of the geriatric nutritional risk index to assess the nutritional status and predict the outcome of home-care resident elderly: a comparison with the mini nutritional assessment. Br J Nutr. 2009;102(4):563-570.
40. Visvanathan R, Chapman I. Undernutrition and anorexia in the older person. Gastroenterol Clin North Am. 2009;38(3):393-409.
41. Payette H, Boutier V, Coulombe C, Gray-Donald K. Benefits of nutritional supplementation in free-living, frail, undernourished elderly people: a prospective randomized community trial. J Am Diet Assoc. 2002;102(8):1088-1095.
42. Thomas D, Zdrowski C, Wilson M, et al. Malnutrition in sub-acute care. Am J Clin Nutr. 2002;75(2):308-313.
43. Hajjar R, Kamel H, Denson K. Malnutrition in aging. The Internet Journal of
Geriatrics and Gerontology. 2004;1(1).










