The Role of Alternative Therapies in the Management of Alzheimer’s Disease and Dementia, Part I
INTRODUCTION
Potential alternative strategies mentioned in the medical literature for the prevention and treatment of Alzheimer’s disease and/or dementia include the following categories of agents and processes: immunization, aggregation inhibitors, secretase inhibitors, transition metal chelators, growth factors, herbs, nonsteroidal anti-inflammatory drugs (NSAIDs), antioxidants, lipid-lowering agents, antihypertensives, selective phosphodiesterase inhibitors, and vitamins (E, B12, B6, C).1 Part I of this article will discuss common alternative therapies currently in use, and the most recent peer-reviewed evidence that does or does not support their use. This will include the following therapies: NSAIDs (including cyclooxygenase-2 [COX-2] inhibitors and aspirin), ginkgo, vitamins (C, B12, folic acid, E), and therapies influencing serum homocysteine levels and selegiline.
Prior to considering the use of any agent, prescription or nonprescription, the treating physician is reminded that reversible cognitive dysfunction secondary to prescription medications and even depression must be ruled out (pseudodementia). Commonly prescribed medications may cause cognitive dysfunction to a mild degree, including amitriptyline, imipramine, propranolol, clonidine, high doses of antipsychotic medications (especially highly anticholinergic and atypical agents), long-acting benzodiazepines, barbiturates, phenytoin, NSAIDs, neuroleptics, H2 blockers (in high doses), narcotics (anticholinergic), gabapentin, carbamazepine, digoxin, corticosteroids, and some antibiotics.2 If possible, reduction or elimination of the agent in question may result in a significant improvement in cognition.
NSAIDs, ASPIRIN, AND COX-2 AGENTS
Before discussing a potential benefit of NSAIDs for Alzheimer’s disease or dementia, it is important to note that these agents may cause gastro-intestinal bleeding, renal insufficiency, and, in rare cases, renal failure.3 As mentioned earlier, case reports have documented that ibuprofen, in particular, is associated with cognitive dysfunction, and that, when discontinued, results in improved cognitive function.4
A global cross-sectional, longitudinal epidemiologic analysis in a Swedish population-based sample from 1991-2000, evaluated clinical, cognitive, and drug treatment data on 702 individuals age 80 years or older.5 The study involved 351 twin pairs of the same sex and evaluated the associations between cognition and the use of aspirin, NSAIDs, paracetamol, or D-propoxyphene. After controlling for age, gender, and cardiovascular and cerebrovascular diseases, patients taking high doses of aspirin had a significantly lower prevalence of Alzheimer’s dementia and better cognitive function than those not taking aspirin. There was a nonsignificant association between the use of low-dose aspirin or NSAIDs and a lower prevalence of Alzheimer’s disease, but not for those taking paracetamol or D-propoxyphene.
A random population sample of 647 Australian patients age 75 years or older was recruited and classified by consensus using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnoses.6 Drug classes administered to the seniors were evaluated for their association with different types of dementia. A total of 163 were found to have dementia, with 78 having Alzheimer’s disease, 45 having vascular dementia, and 40 having other dementia diagnoses (exclusive of Alzheimer’s disease or vascular dementia). These results were compared to 373 controls. Fifty drugs or drug groups were subjected to a chi-square analysis. There was an inverse association between NSAID and aspirin use and Alzheimer’s disease, but not relative to vascular dementia. There was no dose-effect association. Angiotensin-converting enzyme inhibitors also showed an inverse association with Alzheimer’s disease.
In a prospective, population-based cohort study in the Netherlands, 6989 subjects age 55 years or older without dementia at baseline in 1991, were followed with screening in 1993, 1994, 1997, and 1999 to evaluate the association between the use of NSAIDs and Alzheimer’s disease and vascular dementia.7 The risk of Alzheimer’s disease was determined by review of pharmacy documentation. Four categories of NSAID use were defined: nonuse; short-term use (for 1 month or less of cumulative use); intermediate use (more than 1 but less than 24 months of cumulative use); and long-term use (24 months or more of cumulative use). The analysis was adjusted for the following variables: age, sex, education, smoking status, use or nonuse of salicylates, histamine H2 receptor antagonists, antihypertensive agents, and hypoglycemic agents. After a period of 6.8 years follow-up, there were 293 cases of Alzheimer’s disease and 56 cases of vascular dementia. There was no risk of Alzheimer’s disease with short-term use of NSAIDs, a relative risk of 0.83 with intermediate use, and a relative risk of 0.2 with long-term use. There was no association between the use of NSAIDs and vascular dementia.
Indomethacin is one of the oldest NSAIDs, and also appears to be the most neurotoxic agent, since it crosses the blood-brain barrier. For this reason, it is considered to be an inappropriate drug for use in the elderly.8 A Register of the Cochrane Dementia and Cognitive Improvement Group performed a peer review of the literature in 2001 evaluating indomethacin use in single or multicenter, placebo-controlled, randomized trials in patients with Alzheimer’s disease.9 Only one study was selected that met the criteria for review. In this study comparing patients receiving indomethacin to placebo (Rogers 1993), there was no statistical difference between the two groups for the individual cognitive tests, including the Mini-Mental State Examination (MMSE), the Alzheimer’s Disease Assessment Scale (ADAS), the Boston Naming Test (BNT), and the Token Test (TT). There was an increased risk of gastrointestinal bleeding in the indomethacin-treated group. The conclusion of the study was that indomethacin could not be recommended for the treatment of mild-to-moderate Alzheimer’s disease at a dose of 100-150 mg daily.9 A similar review of randomized, double-blind, and placebo-controlled trials of the Cochrane Database of Systemic Reviews was conducted in 2002 assessing the efficacy of ibuprofen for Alzheimer’s disease. Only one trial for age-associated memory impairment was in process but unfinished. Another trial evaluating the effect of ibuprofen on cerebrospinal fluid beta-amyloid in non–cognitively impaired individuals is in process.10
A multicenter, randomized, double-blind, placebo-controlled, parallel group trial with a 1-year exposure to study medications enrolled patients from December 1999 to November 2000.11 The study involved 351 patients with mild-to-moderate Alzheimer’s disease (MMSE scores, 13-26). They were followed for a 1-year change while taking naproxen 220 mg twice daily, rofecoxib 25 mg daily, or placebo. The Alzheimer’s Disease Assessment Scale-Cognitive Subsection (ADAS-Cog) score was used to assess cognitive changes over time. Participants taking stable doses of cholinesterase inhibitors, estrogen, low-dose aspirin, and vitamin E were allowed to continue these therapies during the study period. There was no difference in ADAS-Cog scores between patients treated with naproxen or rofecoxib compared to placebo.11
With regard to the association between aspirin use and vascular dementia, a retrospective analysis of patients treated and untreated with aspirin in North London involved a medical record review of hospital charts and telephone interviews with general practitioners, social service agencies, and institutions (discharged from January 1, 1995 to December 31, 1997).12 The study indicated a trend toward increased time to institutionalization (39 vs 22 months) and a significant reduction in death (71 vs 27 months) for patients taking aspirin versus nontreated patients. A Cochrane review of all randomized, controlled trials evaluating the use of aspirin for vascular dementia was performed in 2000.13 Trials were evaluated for quality of design and risk of bias. No trials were found that were eligible for inclusion in the review. To answer the question regarding low-dose aspirin for prevention of major adverse cardiovascular events and vascular dementia in the elderly, the ASPREE [ASPirin in Reducing Events in the Elderly], a placebo-controlled trial, is in progress as of 2003, which will follow 15,000 subjects age 70 years or older for an average of 5 years.14
VITAMINS AND SELEGILINE
Studies indicate conflicting results regarding diet and vitamin use and Alzheimer’s disease/dementia. To assess the relationship between antioxidant supplement use (multivitamins, B-complex vitamins, vitamins C and E) and Alzheimer’s disease, a cross-sectional, prospective study of elderly patients age 65 years or older in a county in Utah was performed in 1995 and 1997 to detect the prevalence of dementia, with follow-up between 1998 and 2000 to detect incident cases of dementia.15 The study involved 4740 participants, of which 200 cases of prevalent Alzheimer’s disease were identified at initial assessment, and 104 incident cases of Alzheimer’s disease were found at follow-up. Diagnosis of Alzheimer’s disease was made by means of multistage assessment procedures. Combined use of vitamin E and C supplements was significantly associated with a reduced prevalence and incidence of Alzheimer’s disease, and a trend toward significance was noted for patients taking vitamin E and multivitamins containing vitamin C. There was no association noted between the use of vitamin E, vitamin C, multivitamins, or vitamin B-complex supplements alone relative to prevalent or incident cases of Alzheimer’s disease.15
A prospective study in the Washington, DC, area investigated the association between Alzheimer’s disease and intake of carotenes, vitamin C, and vitamin E in 980 elderly participants who were initially free of dementia at baseline and were followed for 4 years.16 Semiquantitative food frequency questionnaires were administered at baseline and at follow-up. Quartiles of each vitamin intake were compared to incident cases of Alzheimer’s disease with adjustment for age, level of education, sex, apolipoprotein E (ApoE) epsilon4 status, ethnicity, and smoking status. At follow-up, 242 incident cases of Alzheimer’s disease had occurred. There was no association found between any quartile of vitamin intake and risk of Alzheimer’s disease.
A Cochrane review was performed in 2000 searching for all unconfounded, double-blind, randomized trials in which treatment with vitamin E at any dose was compared to placebo for patients with Alzheimer’s disease.17 Only one study was found that met inclusion criteria. The study by Sano in 1997 involved 341 participants followed for 4 years, with endpoints including death, institutionalization, loss of two out of three basic activities of daily living, or severe dementia. Severe dementia was defined as a global Clinical Dementia Rating (CDR) of 3. Due to low numbers, it was impossible to determine outcomes and therefore interpret results. Unexpectedly, more participants taking vitamin E sustained a fall. 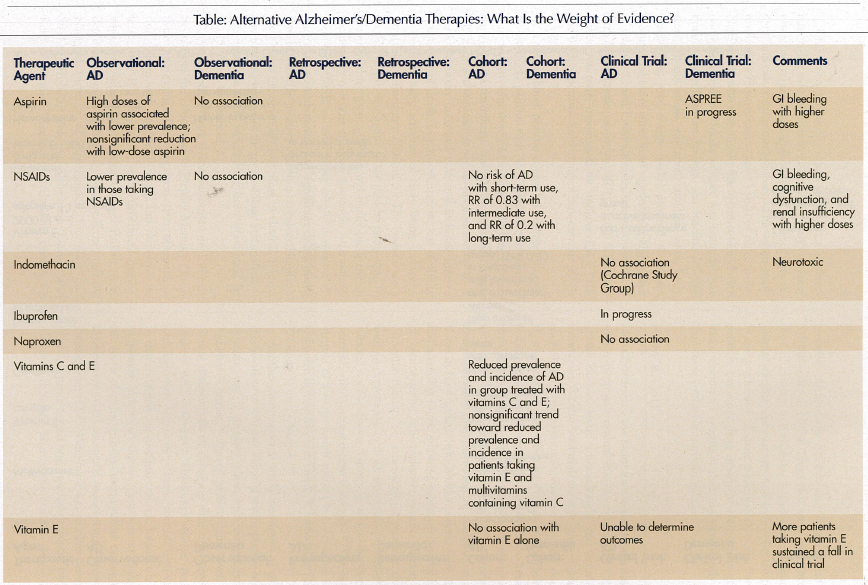
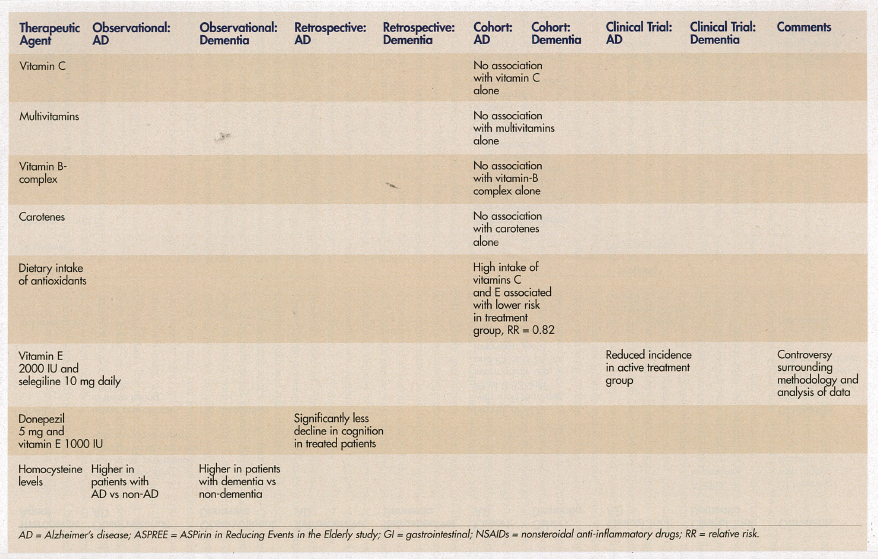
Though conventional wisdom states that a Mediterranean diet reduces the risk of dementia,18 only one study was found on the association of dietary intake of antioxidants and risk of Alzheimer’s disease.19 This cohort study involved 5395 participants, age 55 years or older in the Netherlands, who were initially free of dementia and were noninstitutionalized. Intake of diet was recorded over time. After adjustment for age, sex, baseline MMSE score, alcohol intake, education, smoking status, body mass index, total energy intake, presence of carotid plaques, and use of antioxidant supplements, high intake of vitamins C and E was associated with a lower risk of Alzheimer’s disease (relative risk [RR] = 0.82). This association was more pronounced for smokers (RR = 0.76 and 0.58, respectively) and intake of beta-carotene (RR = 0.54).
A double-blind, placebo-controlled, multicenter trial involved 341 patients with Alzheimer’s disease of moderate severity who were taking either selegiline (10 mg daily), alpha-tocopherol (2000 international units [IU]), or a combination versus placebo for up to 2 years.20 Primary outcomes evaluated included time to occurrence of death, institutionalization, loss of ability to perform basic activities of daily living, or severe dementia (defined as a CDR of 3). The baseline score on the MMSE was higher in the placebo group than in the other three groups, which was highly predictive of the primary outcome of severe dementia. After adjustment for MMSE, patients taking selegiline, alpha-tocopherol, or a combination had significant delays in the time to the other primary outcomes, compared to placebo. Due to the unusually high dose of vitamin E used, and to issues relative to the methodology and analysis of the data, the study results have been questioned by many experts in the field.21
A retrospective chart review performed on 130 participants at the Ohio State University Memory Disorders Clinic evaluated the long-term effects of combination therapy with donepezil (5 mg) and vitamin E (at least 1000 IU) in patients with Alzheimer’s disease, who were followed for at least 1 year.22 Patients were determined to have Alzheimer’s disease if they met the National Institute of Neurological Disorders and Stroke (NINDS) and the Alzheimer’s Disease and Related Disorders Association (ADRDA) criteria for probable Alzheimer’s disease, and if they had an MMSE score of 10-24. These data were compared to the Consortium to Establish a Registry for Alzheimer’s Disease database for patients, and were collected prior to the availability of these treatment options. Participants in the active drug treatment comparison group had a decline in cognition that was significantly less than those in the Consortium group.
HOMOCYSTEINE
Homocysteine is a risk factor for coronary artery disease, stroke, peripheral arterial disease, extracranial carotid arterial disease, aortic atherosclerosis, and deep vein thrombosis. Randomized trials are in progress evaluating multivitamins with folic acid, vitamin B12, and vitamin B6 to determine their effect on the risk of atherosclerotic vascular disease. There is also an association between dementia and possibly Alzheimer’s disease and homocysteine levels.23 This association was further quantified in a cross-sectional study involving an academic nursing home in New York, in which older patients were classified according to the following subgroups and by levels of homocysteine: atherosclerotic vascular disease plus dementia (Group 1); atherosclerotic vascular disease without dementia (Group 2); dementia without atherosclerotic vascular disease (Group 3); and no dementia or atherosclerotic vascular disease (Group 4).24 The highest quartile of average serum plasma homocysteine levels was noted in the patients in Group 1, followed by Group 2, Group 3, then Group 4.
Another cross-sectional study attempted to answer the question of whether elevated serum plasma homocysteine was a primary cause or consequence of Alzheimer’s disease.25 Plasma homocysteine levels and clinical deterioration of dementia were determined in patients with early-onset Alzheimer’s disease, patients with late-onset Alzheimer’s disease, patients with vascular dementia, and age-matched controls. In patients with early-onset Alzheimer’s disease, there was no change in the levels of either plasma homocysteine or cognitive deterioration when compared to age-matched controls. Patients with mixed Alzheimer’s and vascular dementia, as well as patients with only vascular dementia, had significantly increased plasma homocysteine levels compared to controls. Plasma homocysteine levels were elevated in patients with late-onset Alzheimer’s disease with a history of additional cardiovascular disease, as compared to patients with Alzheimer’s disease without such a history and to controls.
A similar study examined the relationship between plasma homocysteine levels and scores on the MMSE.26 Fasting homocysteine levels were measured in 650 cognitively normal Italian community-dwelling patients age 65 years or older. Socioeconomic status, serum folate, vitamin B12, creatinine, other potential dietary and lifestyle determinants of homocysteine, and conventional vascular disease risk factors were also assessed. Those subjects with MMSE scores of 26-28 had higher plasma homocysteine levels than did those with scores higher than 28. Those with scores of 24-25 had higher levels than did subjects with scores of 26-28. The results did not change after adjustment for conventional risk factors or for age, medical, dietary, or lifestyle determinants mentioned above.
White matter lesions and silent brain infarcts are frequently seen on magnetic resonance imaging (MRI) in healthy older people. Both are also associated with an increased risk of stroke and dementia. The association between elevated total homocysteine levels and silent brain infarcts and white matter lesions was studied in a cross-sectional design, part of the Rotterdam Scan Study, a population-based study of 1077 individuals age 60-90 years who had a cerebral MRI performed.27 This relationship was analyzed with adjustment for cardiovascular risk factors. The risk of silent brain infarcts, the severity of periventricular white matter lesions, and the extent of subcortical white matter lesions increased with increasing total homocysteine levels. The associations between white matter lesions still existed after excluding those with silent brain infarcts. Another analysis of this study group involved the examination for the association of serum homocysteine levels and cognitive function cross-sectionally.28 Cognitive performance was assessed and compound scores were constructed for global cognitive function, memory function, and psychomotor speed. Increasing serum homocysteine levels were associated with lower scores for these variables, and most of the association occurred at the highest quintile of homocysteine levels. The results were not mediated by structural brain changes on MRI.
A longitudinal study of 314 consecutive elderly subjects, of which 228 were eligible for analysis, evaluated the relationship of mild cognitive impairment, Alzheimer’s disease, and vascular dementia with total serum homocysteine, folate, and vitamin B12 levels. The levels of these were correlated in 55 patients without dementia, 81 mildly cognitively impaired patients, and 92 patients with dementia with a clinical diagnosis of Alzheimer’s disease or vascular dementia, mostly in the mild stages. Subjects with the lowest folate tertile had significantly higher risks of mild cognitive impairment and dementia. Subjects with the highest serum homocysteine tertile had the lowest MMSE score.29
Finally, a study of 97 individuals with Parkinson’s disease of 3.6 years average duration completed the Beck Depression Inventory, a battery of 11 cognitive tests, and the motor and function components of the Unified Parkinson’s Disease Rating Scale (UPDRS).30 Test results were compared in 66 subjects with normal homocysteine levels and 31 subjects with elevated levels, and were controlled for age, sex, disease duration, and treatment. Patients with elevated homocysteine levels were slightly older but had similar plasma concentrations of vitamin B12 and folate. These patients were also more depressed and performed worse on neuropsychometric tasks compared to those with normal levels.
A cross-sectional study evaluated the effect of hormone replacement therapy (HRT) on serum homocysteine levels and cognitive functioning.31 Serum levels of homocysteine, HRT status, the Modified Mini-Mental State Examination (3MSE), and the DELayed Word RECall test (DELREC) were measured in 1041 elderly postmenopausal women of Latino background. An analysis adjusting for age, education, income, acculturation, and hysterectomy status indicated that those subjects who were prescribed HRT had significantly higher 3MSE and DELREC scores, and higher homocysteine levels than those not prescribed HRT.31
DISCUSSION
Despite all of the “hype” about alternative therapies and their common use by the lay public amounting to a multi-million dollar business, there appears to be little evidence-based medicine that justifies use of specific agents.
Specifically, despite cross-sectional and longitudinal studies indicating that NSAIDs are associated with a lower risk of Alzheimer’s disease or vascular dementia, randomized trials of various traditional agents and COX-2 inhibitors have failed to validate these findings to date. On the contrary, high doses may be associated with cognitive dysfunction. There is also little data to support the use of aspirin for vascular dementia. Other than vitamin E in a dose of 2000 IU, there is no definitive evidence that vitamin supplementation is effective in the prevention of dementia. Relative to B12 and folate, there appears to be, at best, an association between elevated homocysteine levels and dementia or cognitive impairment.
As might be expected, there appears to be a significant amount of information in the literature on the association between various alternative therapies and Alzheimer’s disease or dementia. These preliminary findings from case-control or restrospective studies, as noted above, are seldom proven to be accurate when tested by the gold standard of research designs—the double-blind, placebo-controlled clinical trial format. Unfortunately, there appears to be few of these well-done clinical trials relative to these therapies. To some extent, this is due to the expense, time, expertise, and large populations of participants necessary to undertake such endeavors. Although these studies are necessary since they have the potential to do harm as well as provide benefit, the over-the-counter nature of these agents—and therefore ready access to the public—makes them a much less attractive option for pharmaceutical companies. It is likely that validation of these findings will be slow in coming, from academic medical centers with a special interest in such topics and with grant support provided by nonprofit or state or federal grant programs.
Part II of this article, which will appear in the August issue of the Journal, will examine herbals, hormones, statins, alcohol, exercise, and socialization.










