Pharmacologic Management of Chronic Obstructive Pulmonary Disease in the Elderly
Chronic obstructive pulmonary disease (COPD) is a progressive inflammatory disorder that is usually diagnosed in one’s middle age or later and worsens with time. Thus, elderly persons with COPD often present challenges of severe respiratory compromise, frequent exacerbations, common nonrespiratory comorbidities, and poor quality of life due to these illnesses. International guidelines for diagnosing and managing COPD have been developed, and the most often cited of these is the Global Initiative for Chronic Obstructive Lung Disease (GOLD).1 Recently, patient and physician surveys suggest that inadequate knowledge and poor adherence to these guidelines, along with insurance impediments, adversely affect the quality of care of persons with COPD.2 This review will focus on the pharmacologic management of COPD, with specific reference to those persons over age 65 and/or residing in long-term care facilities.
GOALS OF TREATMENT
Once established, COPD is not curable, so the goals of therapy are to:
• Slow disease progression
• Relieve symptoms with minimal medication side effects
• Prevent and treat exacerbations
• Improve exercise tolerance
• Prevent and treat complications
• Improve health status
• Reduce mortality1
The following discussion reviews the pharmacotherapy needed to attain each goal.
Slow Disease Progression
COPD progression is measured with spirometry and determined by the decline in Forced Expiratory Volume (FEV1) over time. Around age 25, all persons have declining lung function, as noted by the slope of the FEV1 versus age curve. Those without pulmonary compromise decline at roughly 15-30 mL/year. Individuals with COPD, however, have a much steeper slope that leads to considerable disability at a much younger age.3 A number of investigators have tried to improve on this natural course of COPD with various pharmacologic agents. The Lung Health Study4 and Lung Health Study II5 explored whether inhaled ipratropium or triamcinolone, respectively, could favorably alter this slope. Unfortunately, the decline in lung function remained unchanged after treatment with these medications.
The only treatment thus far proven to change the course of lung function decline is smoking cessation. In the original Lung Health Study, the smoking cessation intervention groups experienced significantly smaller declines in FEV1 than the control group over a period of 5 years.4 So, while often difficult to achieve, smoking cessation should be the main emphasis to achieve the goal of slowing COPD progression, even in the elderly.
To be successful, individuals have to be ready and motivated to quit smoking, and then the proper support should be available to assist in the process. One might think that elderly smokers would be unwilling to quit after many years of smoking. In fact, older smokers quit smoking at rates comparable to younger smokers when they are provided with appropriate tools, and significant health benefits can accrue to elderly smokers who quit.6 As with younger patients, elderly smokers should be encouraged to set quit dates and learn coping strategies for triggers.
Policies may facilitate behavior change, and smoking policies in nursing homes may be less restrictive than other healthcare facilities in an effort to balance the residents’ rights and the institution’s goals.7 A survey of nursing staff at a long-term care facility demonstrated that the staff’s perception of residents’ unwillingness to quit and lack of institutional support for assisting in cessation were main barriers to advising them to quit. In addition, the nurses questioned whether they had the skills to assist residents in a quit attempt.7 Thus, opportunities exist for improving how long-term care facilities approach smoking cessation, but they may require a cultural shift toward stricter policies and staff training.
Medications should be considered to minimize withdrawal symptoms while undergoing a quit attempt. Several first-line products for smoking cessation are available, with specific considerations for the elderly. Five nicotine replacement products are currently on the market including gum, lozenges, patches, nasal spray, and oral inhaler. For complete prescribing information for these products, the reader is referred to the manufacturers’ package inserts. Although nicotine replacement products may list cardiovascular disease as a precaution, this comorbidity should not be considered a contraindication, as these products have been shown to be safe with underlying cardiovascular disease.8 Elderly persons with dentures may find the gum difficult to use. Since depression is a common comorbidity with COPD, bupropion may offer an advantage of possibly treating depression while being used for smoking cessation.9,10 Varenicline, a nicotinic acetylcholine receptor partial agonist, was recently approved, and requires dosage titration to minimize the most common side effect of nausea.11 While combining therapies is reasonable for highly addicted individuals (eg, bupropion plus nicotine replacement), combining varenicline with nicotine replacement increases the incidence of nausea and other adverse effects.11
Relieve Symptoms
Bronchodilators are the agents of choice for improving the symptoms of dyspnea, chest tightness, and shortness of breath. The categories of bronchodilators include beta2-agonists, anticholinergics, and theophylline. Beta2-agonists and anticholinergics can be further broken down into short-acting and long-acting agents. The GOLD guidelines suggest that short-acting agents be used as initial bronchodilators in mild COPD (FEV1 ≥ 80%) with intermittent symptoms, and that long-acting bronchodilators be the core therapy in more severe COPD.1 Thus, many elderly persons would likely be candidates for long-acting bronchodilators as maintenance therapy while using short-acting agents as needed for acute symptom control. A recent Cochrane review of eleven trials comparing ipratropium and short-acting beta2-agonists concluded that while ipratropium offered some slight advantages, there could not be a general recommendation for the use of ipratropium or a combination of ipratropium and a beta-agonist over the beta agonist alone.12 The GOLD guidelines suggest that the main consideration for choosing one product over the other is individual response and preference.1 When a single long-acting bronchodilator does not adequately control symptoms, adding a long-acting bronchodilator from the other class is recommended.
Short-acting beta-agonists. Albuterol is the most commonly prescribed short-acting beta2-agonist (SABA), although other choices include pirbuterol, metaproterenol, and levalbuterol (Table I). All four agents have comparable durations of action and frequency of side effects. Therefore, route of administration, patient preference, and costs may be the main considerations for selecting a specific agent. While intermittent as-needed use of SABA is recommended in asthma, regular use is recommended in COPD.13 Although there are oral products available for some of these agents, inhaled therapy is preferred to minimize adverse effects unless one cannot take inhaled agents. The main adverse effect of a SABA is tremor with high doses, and the elderly are more prone to this.1 Heart rhythm disturbances are rare.
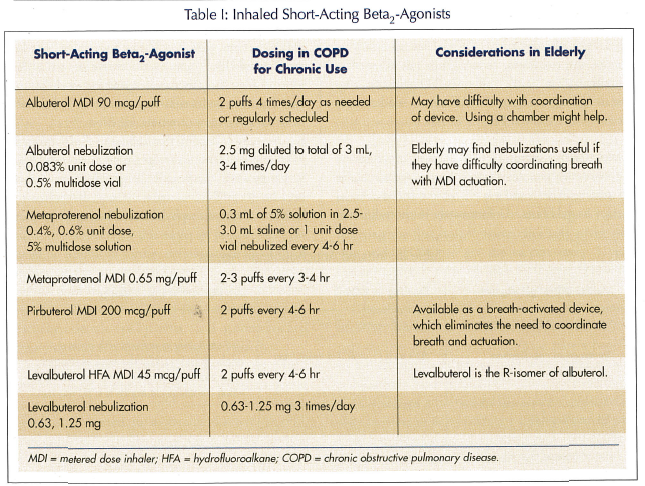
Long-acting beta-agonists. Salmeterol and formoterol are two long-acting beta2-agonists (LABAs) that allow less-frequent dosing than SABAs, with comparable improvements in lung function (Table II). Formoterol has a more rapid onset of action than salmeterol (5 min vs 20 min, respectively). Whether this is a clinically significant advantage for a drug administered routinely for maintenance therapy is debatable. Other than onset of action, both agents are comparable in efficacy and side effects in COPD.14 Both of these agents are available as dry 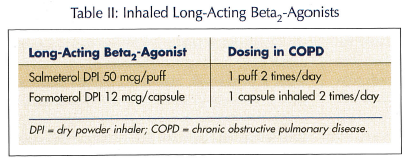 powder inhalers (DPIs) that require an adequate inspiratory flow rate to get the drug out of the inhaler device and into the lungs. Fortunately, both of the devices deliver adequate amounts of drug at inspiratory flow rates achievable by most persons with COPD.
powder inhalers (DPIs) that require an adequate inspiratory flow rate to get the drug out of the inhaler device and into the lungs. Fortunately, both of the devices deliver adequate amounts of drug at inspiratory flow rates achievable by most persons with COPD.
These agents have an excellent safety record, with adverse effect rates similar to placebo in most studies. It is important to note that salmeterol has a black box warning in its prescribing information, but it is related to the treatment of asthma,15 and does not imply the same caution in treating COPD. Unlike asthma, where monotherapy with a LABA leads to deterioration of the condition, these agents can be used as monotherapy in COPD. The GOLD guidelines suggest using long-acting bronchodilators in moderate-to-severe COPD (50% ≤ FEV1 < 80%).1
Anticholinergic agents. Until recently, ipratropium was the only widely prescribed anticholinergic agent available for treating COPD, and it is still recommended as initial therapy in mild cases. Unfortunately, its short duration of action of 6 hours requires multiple daily administrations. In 2004, tiotropium was the first long-acting anticholinergic agent approved by the Food and Drug Administration (Table III). Tiotropium has a duration of action of 24 hours, thus allowing once-daily administration. As with the LABA, tiotropium should also be considered a treatment of choice for moderate-to-severe levels of COPD. The GOLD guidelines give no preference for tiotropium or the LABA for treating COPD. Some authors, however, suggest that tiotropium has a slight edge in overall efficacy over salmeterol.13,14 In one 6-month comparison, tiotropium produced better bronchodilation, dyspnea scores, and health-related quality of life scores than salmeterol.16 While caution is advised regarding ocular effects of anticholinergics, it has been proposed that such effects are due to topical absorption rather than systemic action, perhaps from errant spray from a metered dose inhaler (MDI).17 Thus, a product like tiotropium, delivered by a DPI, would likely carry a very low risk for such adverse effects, unless the person had significant renal dysfunction that increased serum concentrations. Dry mouth, however, is the most common side effect.18
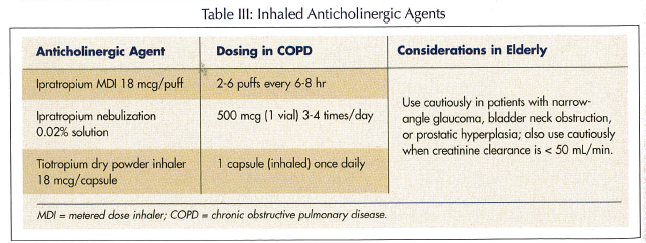
One note of caution that applies to the ipratropium MDI and the combination ipratropium/albuterol MDI is that persons with peanut allergy should avoid them because the products contain a peanut derivative, soya lecithin. Cases of anaphylaxis after taking ipratropium aerosol have been reported.19 This does not apply to the nebulization solution or the nasal spray.
Combination bronchodilators. The combination of bronchodilators with different mechanisms may add benefit over one bronchodilator alone, with few adverse effects. However, there is still the need for frequent dosing (Table IV). If using the long-acting bronchodilators, one might begin with either a LABA or tiotropium and then add the other class if adequate relief is not obtained with the initial therapy.

Theophylline. While once widely prescribed for COPD, theophylline is now considered an add-on therapy for persons who are not well-controlled on the long-acting bronchodilators noted above. While effective, the potential for adverse effects and drug interactions, as well as the need for serum concentration monitoring, have limited the current use of theophylline in favor of effective but safer products. Clearance is reduced in the elderly, and many drugs inhibit the metabolism of theophylline. Thus, careful dose titration is encouraged when such drugs are added or deleted. Because theophylline produces adverse effects even within the usually declared therapeutic range of 10-20 mcg/mL, yet produces beneficial effects at concentrations lower than this, a re-evaluation of this target in prospective trials would be helpful in further defining the role of theophylline in managing COPD.20 Theophylline blood levels of 8-12 mcg/mL may provide benefits with less toxicity. Signs of theophylline toxicity include headache, tremor, tachycardia, and gastrointestinal symptoms such as nausea, vomiting, and diarrhea.
To summarize, bronchodilators are the main agents used for symptom control. The short-acting agents may be used in mild cases, but long-acting agents are preferred in moderate-to-severe COPD. Combining bronchodilators of different classes is preferred when one agent alone does not provide adequate relief of symptoms.
Prevent and Treat Exacerbations
COPD exacerbations may impair one’s health status for months and produce a heavy burden on the healthcare system. Preventing these episodes is a key strategy for long-term care facilities. Annual influenza vaccination and at least one vaccination with the 23-valent pneumococcal polysaccharide vaccine (PPV) are important approaches for the prevention of COPD exacerbations. There is controversy over how often someone with COPD should receive the pneumococcal vaccine. According to the Centers for Disease Control and Prevention (CDC), persons should receive only one dose if they are over age 65 when receiving the first dose. Those with chronic lung diseases such as COPD should receive their first dose anytime between ages 2 and 64 years. Revaccination with one dose is recommended if at least 5 years have elapsed since the first dose, and if the person was under age 65 when receiving the first dose.21 Some clinicians advise persons with COPD to get the vaccine every 5 years because antibody titers fall after 5-10 years. However, the need for multiple revaccinations is still unclear.
The efficacy of PPV for preventing community-acquired pneumonia (CAP) in persons with COPD may decline with age, but some have shown benefits of the vaccine in elderly persons as it relates to hospitalizations and mortality.22 Since persons with CAP are often admitted to the hospital, this setting may be ideal to administer the PPV to persons who have not received it. Long-term care facilities may also be ideal locations to ensure adequate immunization of residents.
It appears that several drugs or drug combinations that are used for the clinical improvement in COPD symptoms and lung function may also reduce exacerbation rates. For example, both salmeterol and formoterol have been shown to reduce exacerbation rates, as has tiotropium. Data are less clear on the effect of short-acting bronchodilators on exacerbation prevention. Inhaled steroids alone and combinations of inhaled steroids and LABAs have also reduced exacerbation rates.23, 24 The GOLD guidelines suggest that inhaled steroids be used in persons whose FEV1 is less than 50% (severe-to-very severe COPD) and who experience frequent exacerbations (eg, 3 in the last 3 yr).1 As pointed out by Scott et al,25 the optimum product or combination has yet to be defined and may depend on the individual’s phenotype. The clinical trials report population average data and provide important guidance to therapy, but the heterogeneous COPD population requires an individualized approach. Inhaled steroid use in COPD has often been disappointing when looking for lung function improvements. However, newer data looking at exacerbation rates and humanistic outcomes appear more promising.
To summarize, for preventing exacerbations, ensuring annual influenza vaccination and at least one pneumococcal vaccination are important steps. Standing-order procedures in nursing homes are encouraged, yet a small percentage of such facilities use them.26 In addition, using the long-acting bronchodilators and inhaled steroids (in the severe population) may reduce exacerbation rates in comparison to short-acting bronchodilators.
Once a person experiences an acute exacerbation of COPD (AECOPD), management depends on the severity of the exacerbation. Elderly persons are often hospitalized for exacerbations. In addition to oxygen therapy or mechanical ventilation, the main pharmacologic classes to implement are bronchodilators, systemic steroids, and antibiotics. Unlike maintenance therapy, acute therapy requires short-acting bronchodilators, and beta2-agonists are preferred.1 Albuterol can be administered by nebulization or MDI with or without a spacer/chamber. A nebulized albuterol dose of 2.5 mg every 4 hours until recovery was shown to provide equal outcomes to a 5-mg dose in persons with moderate nonacidotic AECOPD.27 Direct comparisons of MDIs plus chambers versus nebulizers have come from the asthma literature and consistently show that either device yields similar outcomes. Dolovich et al28 reviewed the literature on devices and provides evidence-based guidelines on selection of inhalation delivery method. Their recommendations suggest that either the nebulizer or MDI plus spacer/holding chamber are appropriate in the inpatient setting and should provide equivalent outcomes. Some considerations include the person’s ability to use a device correctly, the person’s preference, costs, and drug/device availability. Many elderly individuals may have difficulty with self-administration of medication via MDIs, and a breath-activated device may be preferred.29 Since neither DPIs nor MDIs without spacers/holding chambers have been compared in this setting, no recommendations were made for them. However, their use in the outpatient setting may be appropriate.28 In the face of an AECOPD, increasing the dose and/or frequency of short-acting beta2-agonists is appropriate. Adding ipratropium may be necessary without a prompt response to the SABA.
Systemic steroids should be included in the hospital management of AECOPD, and the GOLD guidelines recommend prednisolone 30-40 mg daily for 10-14 days, stating that the best dose is not known (prednisone would have the same dosing).1 While the VA Cooperative study30 demonstrated that a 2-week course of steroids is as effective as 8 weeks, no studies have directly compared various doses for the same duration. In an effort to balance effectiveness with minimal adverse effects, such as psychosis, bone loss, and hyperglycemia, this low-dose approach as recommended by GOLD is appropriate for most persons with AECOPD until further data are available.31 While some clinicians taper the steroids over this 2-week period, this is not necessary.31
The appropriate use of antibiotics in AECOPD has been debated for many years without consensus on when to use them, nor which antibiotic classes are most appropriate. This is partially due to the difficulty in establishing the role of bacteria in a specific case, due to frequent respiratory tract colonization and subtle signs and symptoms of exacerbation in some persons that may be difficult to distinguish from the underlying disease.32 Nevertheless, most exacerbations are treated with antibiotics. The GOLD guidelines recommend antibiotics in persons who have three of the cardinal symptoms (ie, increased dyspnea, increased sputum volume, and increased sputum purulence). Only two of the cardinal symptoms have to be present when increased purulence is one of them.1 Antibiotic selections are determined by pathogens and local resistance patterns. The most common bacterial pathogens in AECOPD include Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Because of increasing resistance patterns of these bacteria, broad-spectrum antibiotics are commonly used, even though there is little data proving their superiority, and nursing home data are lacking. The range of duration of therapy is 3-14 days.
Antibiotic use in the elderly poses distinct challenges. Bacterial flora in nursing homes is typically more resistant than those found in the community, so a resident of a nursing home may need tailored antibiotic therapy; again, little prospective data provide specific guidance. Antibiotic clearance through the kidneys will likely be compromised in elderly persons, and dosage adjustments based on creatinine clearance is prudent.33 For example, the dose of levofloxacin should be reduced by 50% when the creatinine clearance is less than 50 mL/min.33 A system should be in place where product information is consulted for dosage adjustment recommendations in persons with renal impairment. The GOLD guidelines outline specific antibiotic recommendations based on severity of exacerbation.1
There are many possible choices of oral antibiotics for outpatient management of mild exacerbations. These include amoxicillin with clavulanic acid, doxycycline, azithromycin, clarithromycin, and 2nd or 3rd generation cephalosporins. For hospitalized patients who can still be treated with oral agents, amoxicillin/clavulanic acid, and the fluoroquinolones (levofloxacin, moxifloxacin, gemifloxacin) are appropriate choices. Parenteral therapy might include ampicillin/sulbactam, 2nd or 3rd generation cephalosporins, or fluoroquinolones. High-dose levofloxacin (750 mg) is recommended if this agent is used and antipseudomonal coverage is needed.1
Improve Exercise Tolerance
Breathlessness with exercise is a major determinant of impaired quality of life in COPD. Pulmonary rehabilitation is an important component of COPD management and has been shown to improve exercise tolerance, perception of breathlessness, and quality of life for elderly persons with COPD. Individuals at all levels of severity may benefit from pulmonary rehabilitation programs.1 The breathlessness associated with physical activity may be related to dynamic hyperinflation, and some of the medications used to treat COPD can decrease this phenomenon. Specifically, both anticholinergic and beta2-agonist bronchodilators improve end expiratory lung volumes during exercise and feelings of breathlessness.34 Currently, there are not enough data to show preference of one class over the other, or combinations of the classes over single agents for improving exercise tolerance, so managing the symptoms as noted above will likely also improve exercise tolerance.
Prevent and Treat Complications
COPD is now considered to have systemic effects rather than being just localized to the lungs. Many complications of COPD are related to the progression of the disease and have no specific targeted preventive strategies (eg, right-sided heart failure, cor pulmonale). Rather, treating the underlying airflow obstruction and improving oxygenation are the basic treatments for many of these complications. However, osteoporosis is a specific complication that deserves attention and preventive approaches. Since persons with COPD are at increased risk for this bone disorder and its resultant fractures, the need for bone density assessments should be assessed according to risk factors, and appropriate therapies should be initiated to prevent further bone loss. Adequate calcium and vitamin D should be ensured, and the National Osteoporosis Foundation suggests that men and women over 50 years of age receive 1200 mg of calcium. This is the elemental calcium amount, however, and not the labeled amount on the bottle. Calcium carbonate, for example, is 40% elemental calcium, so a 600-mg tablet contains 240 mg elemental calcium. Bisphosphonates may improve bone density scores by a little and fracture rates by much more. Depression and anxiety are very common comorbidities of COPD, and screening for these and treating with appropriate antidepressants and anxiolytics (eg, alprazolam, lorazepam) is suggested. Depression may be somewhat relieved with airflow obstruction improvement and anxiety treatment.
Improve Health Status
While historically, COPD has been considered almost untreatable because of the relative resistance to pharmacotherapy for improving lung function, today there is an emphasis on improving health status or quality of life. Certainly, reducing exacerbations and improving symptoms by the methods described above should improve quality of life. Some studies are specifically looking at these measures, and the most often used validated quality-of-life measurement for COPD is the St. George’s Respiratory Questionnaire (SGRQ). One study showed that the combination of fluticasone plus salmeterol was the only agent that improved the SGRQ to a clinically significant degree when compared to placebo, salmeterol alone, and fluticasone alone.35 More data like these will be important to determine the global impact of various therapies on patients’ lives beyond physiologic measures such as lung function.
Improve Mortality
Currently, long-term oxygen therapy is the only intervention that has been shown to improve mortality in COPD. Long-term oxygen therapy should be considered for individuals who have arterial hypoxemia (resting PaO2 ≤ 55 mm Hg or SaO2 ≤ 88%) or a PaO2 of 56-60 mm Hg (SaO2 89%) with comorbid pulmonary hypertension, cor pulmonale, edema from right heart failure, impaired mental status, or secondary erythrocytosis (hematocrit > 56%).36 Documentation that individuals meet specific criteria will be needed for insurance reimbursement. More than 15 hours per day of oxygen is recommended to correct the hypoxemia and reach a resting PaO2 ≥ 60 mm Hg or SaO2 ≥ 90%. This will then improve pulmonary artery pressures and oxygen delivery to tissues.
Individuals who are hypoxemic at rest should also receive increased levels of oxygen at night and when exercising to achieve the goal PaO2 or SaO2. Generally, increasing the resting rate by 1 liter/minute for sleep and/or exercise will prevent desaturation.36 If necessary, this can be guided by continuous oximetry during sleep or during exercise. Some persons, however, only develop hypoxemia during sleep or exercise, and oxygen may only be administered during these times. Such limited therapy, however, has not been shown to improve survival.
Oxygen is typically delivered via nasal canula at flow rates of ≤ 3 liter/minute, although transtracheal delivery is a potential option for some patients that can decrease the amount of oxygen needed. With nasal canula delivery of 100% oxygen diluted by the volume of inhaled ambient air, each 1 liter/minute of oxygen flow increases the fraction of inspired oxygen (FIO2) by about 3-4%.36 For example, the FIO2 of ambient air is 20.9% oxygen. A patient receiving supplemental oxygen at 1 liter/minute will have an FIO2 of approximately 24%. Oxygen can be delivered by various devices, each with its own advantages and disadvantages. Concentrators for oxygen delivery are the least expensive devices, but are limited to those patients who spend most of their time at home, due to the lack of portability for ambulatory use. These devices pull oxygen from the ambient air and concentrate and store it for delivery. Because these systems run on electricity, patients would need compressed cylinders as backup. Liquid systems are favorable for individuals who spend much of their time out of the home, due to their suitability for ambulatory use. Oxygen may also be delivered by compressed gas cylinders, as well as oxygen-conserving devices that make oxygen delivery more efficient (eg, by delivering oxygen only at the onset of inhalation). With any of these devices, persons must be told of the dangers of smoking around oxygen or storing oxygen near heat or flame sources.
Other than long-term oxygen therapy in appropriate patients, little data support an impact on survival from other therapies. However, a retrospective database study showed improved survival over 3 years with at least 6 months exposure to the combination of fluticasone and salmeterol over either agent alone.37 To clarify the issue, a prospective placebo-controlled study is underway comparing these same agents with all-cause mortality as the primary endpoint.38 When these data are published, we will have a better understanding of the impact of inhaled steroids and long-acting beta2-agonists on mortality.
CFC-Free Inhalers
There has been a government-mandated transition away from MDIs that contain chlorofluorocarbons (CFCs) as their propellant, due to the ozone-depleting capability of the CFC. An alternative propellant known as hydrofluoroalkane (HFA) has begun replacing the CFC propellants in MDIs. DPIs have no propellant at all, and therefore meet the CFC-free requirements. While a specific deadline has not been set for all inhalers to be CFC-free, the deadline for albuterol inhalers has recently been set at December 31, 2008. After this date, only CFC-free albuterol will be available. Individuals should know that CFC-free inhalers have a different feel to the spray that is emitted from the device. It is less forceful and does not have the cold feel to it. The inhaler itself may feel lighter than the CFC-containing inhalers.
CONCLUSION
COPD can be a difficult disease to manage because our best pharmacotherapy does not provide cures or even dramatic improvements in symptoms and physiologic measures. However, now that more data are emerging on the impact on quality-of-life measures, exacerbation rates, and mortality, perhaps our changing paradigm of aggressively pursuing these outcomes will improve our outlook on this relentless condition. Processes can be implemented in long-term care facilities to ensure that preventive measures are in place that may positively impact one’s quality of life. The author reports no relevant financial relationships.










