Latent Tuberculosis Infection: Successful Screening and Treatment of Older Adults in a Program of All-Inclusive Care for the Elderly (PACE)
Abstract: Latent tuberculosis infection (LTBI) is a highly prevalent condition among older adults in long-term care facilities (LTCFs). Despite this, LTBI testing practices and treatment vary across LTCFs, placing residents at risk of acquiring TB and contributing to the potential for a TB outbreak. The heterogeneity of LTBI testing practices and treatment is a result of the ambiguity around the accurate diagnosis of LTBI, the toxicity of treatment regimens, and the high multimorbidity rate of patients in LTCFs. LTBI is a significant public health problem among older adults, hence, identification and treatment of individuals at highest risk of active TB can help to eliminate the condition. A case series is presented of 7 patients who screened positive for LTBI upon enrollment in a Program of All-Inclusive Care for the Elderly in Michigan and who successfully completed treatment at the program.
Key words: latent tuberculosis infection, tuberculin skin test (TST), PACE
Tuberculosis (TB)—infection with Mycobacterium tuberculosis—is a significant public health problem globally and in the United States due to its increased prevalence among persons born outside of the United States. The burden of TB is high in US adults aged 65 years or older, and the yearly TB rates in this age group are 1.5 times higher than in adults aged 21 to 64 years.1 The prevalence of TB is 2.3 times higher in older adult residents of long-term care facilitites (LTCFs) than in community-dwelling older adults.1 Since 83.7% of TB cases among foreign-born persons in the United States are reactivations of LTBI,2 effective screening and appropriate therapy can prevent active TB infections.3
The incidence of TB is higher in older adults due to cumulative exposure and the increased risk of progression to active disease.4-6 Reactivation of LTBI due to declining immunity as opposed to new infection could explain the steady increase in the median age of patients with TB since 1950.7-9
This highlights the importance of accuracy in diagnosis of LTBI in this population, particularly when planning treatment.
Diagnosing active TB poses specific challenges in older adults. Older adults tend to have atypical presentations of active TB with involvement of the meninges, kidneys, and skeletal system, and pulmonary involvement with negative sputum test results.10-12 Older adults with pulmonary involvement typically do not present with the classic symptoms of hemoptysis, dyspnea, and fever. This atypical presentation of active TB underscores the importance of screening older adults in all settings and particularly LTCFs, where an outbreak would have a huge public health and regulatory impact.
Because of the current culture shift toward enabling aging at home instead of in traditional LTCFs, many older adults are able to live independently with additional support services, with added monitoring, or in alternative group environments. This has led to refined programs such as the Program of All-Inclusive Care for the Elderly (PACE), group homes, continuing-care residential communities, and Green House facilities, all of which pose a new challenge in terms of screening protocols for communicable disease such as TB. LTCFs, congregate settings, newer innovative PACE programs, and other group-setting care models are required to screen patients for TB on program admission.
In this case series, the screening and confirmatory tests for LTBI are discussed, as well as the feasibility of the standard 9-month isoniazid and 3-month short-course isoniazid plus rifapentine treatment regimens in 7 patients enrolled in a PACE program.
Background and Design
We present 7 patient cases among 125 patients who screened positive for TB on entry into a PACE program in Washtenaw County, MI. The case rate of TB in Washtenaw county in 2016 was 2.8/100,000.13 PACE programs are Medicare and Medicaid programs that enable older adults who are nursing-home eligible to live in the community. Patients enrolled in PACE programs most often are frail and multimorbid with complex health problems, and their vulnerability to disease reactivation and their lower threshold for acquiring infections such as TB are comparable with those of residents of LTCFs. Most PACE patients attend day health programs and are in close proximity to other residents and health care personnel. Hence, they are required to undergo screening for communicable diseases such as TB on entry to a PACE program.
The study period was from January 2014 to March 2016. All new patients had an initial 2-step tuberculin skin test (TST) as a screening requirement on entry to the PACE program in accordance with Centers for Disease Control and Prevention (CDC) and American Geriatrics Society guidelines for LTBI screening on entry to an LTCF.14-16 Participants who had a positive TST result underwent interferon-γ release assay (IGRA) testing. IGRA positivity was considered confirmatory of LTBI with negative chest radiography findings, and hence treatment for LTBI was initiated.
Among the 7 patients with a positive TST result, 6 patients had confirmed positive results, and 1 patient had negative results on follow-up IGRA testing. For the TST-positive, IGRA-negative patient, we decided to follow up with yearly IGRA testing. The mean age of the participants was 77.9 years with a male to female ratio of 6:1. The testing algorithm adopted for identification and treatment of LTBI in the PACE program is described in the accompanying Figure 1.
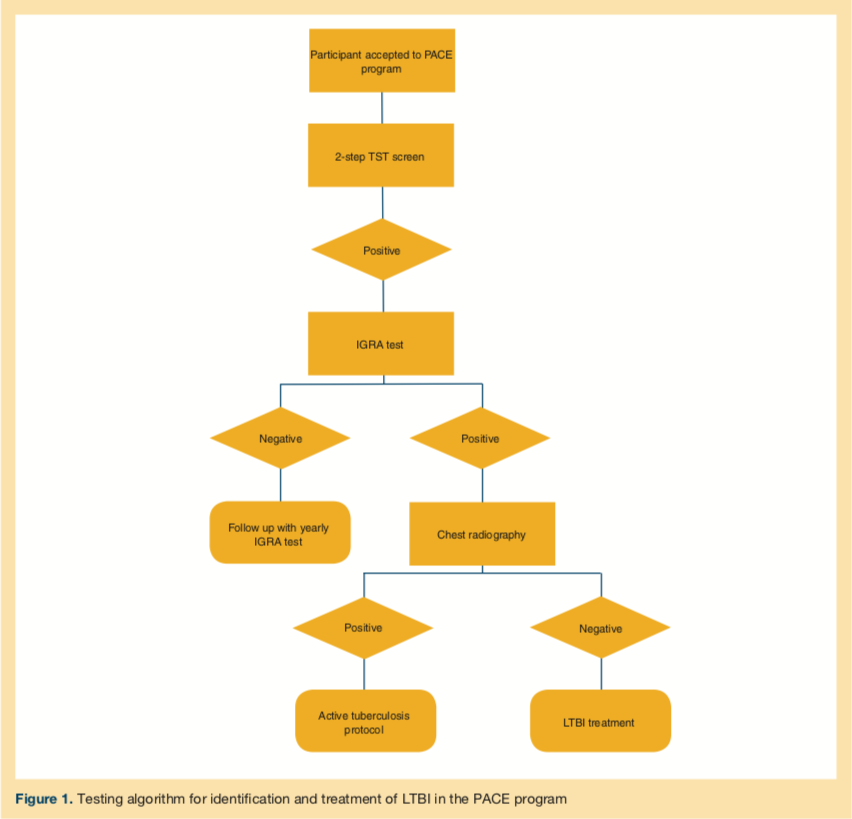
We followed the American Thoracic Society (ATS) and CDC guidelines for LTBI treatment.3,17 In this cohort, 3 patients received therapy with isoniazid once daily for 9 months for a total of 270 doses, and 3 patients received isoniazid plus rifapentine once weekly for 3 months for a total of 12 doses. The TST-positive but IGRA-negative patient was monitored with serial yearly IGRA testing. None of the patients had preexisting liver disease, although one had a history of alcoholism and reportedly remained sober during treatment. All patients were advised to abstain from alcohol while taking isoniazid.
The treatment group of 6 patients was followed monthly in the adult day health clinic at the PACE program to monitor for signs and symptoms of toxicity, including hepatic and hematologic toxicity with laboratory testing. The treatment completion rate was 100%, and there were no reports of peripheral neuropathy. Two patients had mildly elevated liver function test results (less than twice the upper limit of normal) in the isoniazid plus rifapentine arm, but this finding did not interfere with the patients’ completion of the treatment. One patient died from thromboembolic disease, which was determined to be unrelated to LTBI treatment.
The decision to treat the initial cohort of 3 patients with isoniazid monotherapy was due to the unfamiliarity with the short-course regimen. Hence, during the latter part of the study period, as the short-course regimen started to gain popularity, the decision was made to initiate a 3-month course of isoniazid plus rifapentine. We did not note any increase in the number of adverse effects in the isoniazid plus rifapentine cohort.
Discussion
In this assessment of 7 older adult patients, screening for LTBI using the TST or IGRA test, and completion of LTBI treatment, were practical. The cases demonstrate that, in nursing-home eligible, frail older adults, screening for LTBI and treatment with either the conventional 9-month isoniazid regimen or a short-course isoniazid plus rifapentine combination regimen are successful. We also propose that the short-term high-dose combination isoniazid plus rifapentine regimen seems to be more cost-effective, with proven improved treatment completion rates, than the traditional 9-month isoniazid monotherapy regimen. In our small case series, short-course isoniazid plus rifapentine treatment, particularly in the patient with a history of alcoholism, made it easy to enforce alcohol abstinence.
Because treatment of active TB has proven to be successful in older adults when they are able to complete the treatment regimen,10 LTBI treatment is possible with the advent of short-term treatment regimens including high-dose isoniazid plus rifapentine. It should not be underemphasized that individual decision-making on a case by case basis and ongoing monitoring are critical due to age-related pharmacodynamics and comorbidities.
The highlight of the case series was the 100% completion rate and the overall tolerability of conservative and short-course treatment regimens of the older adults. A major contributor to the 100% completion rate with close monitoring was the ease of directly observed treatment and laboratory testing, since the patients were enrolled in a PACE program, which most patients attended weekly. Hence, replication in the primary care outpatient setting would require patient adherence and the associated challenges in completion of treatment and monitoring for adverse effects. In our review, IGRA positivity for LTBI was considered confirmatory, since it exhibits better specificity than the TST and may be preferred as the standard of care in older adults. A limitation of the study is the small number of participants treated successfully (n = 6).
Undiagnosed LTBI reactivation can pose a threat to LTCF residents and employees, resulting in TST conversion and decision dilemmas about the treatment of residents with comorbidities and of employees. Active TB cases have a huge clinical and public health impact due to need for isolation, employee precautions, hospitalization of active cases, and morbidity and mortality of affected individuals. The financial impact on LTCFs and congregational settings parallels the clinical challenges and includes the cost of contact investigation, the cost of treatment of TST converters, and the cost of prolonged lengths of stay for inpatient treatment of active TB.
The World Health Organization, the Infectious Diseases Society of America (IDSA), the ATS, and the CDC recommend screening for LTBI in high-risk groups if treatment is to be considered.18 In 2016, The US Preventive Services Task Force recommended screening for LTBI in populations at increased risk as a B recommendation.19
No gold standard diagnostic test currently exists for screening for LTBI, and current test results provide immunologic evidence of host response to TB antigens.3 The 2 diagnostic tests for LTBI—TST and IGRA—depend on cell-mediated immunity and cannot help distinguish LTBI from active TB disease.20,21 Two types of IGRAs are currently approved by the US Food and Drug Administration: the QuantiFERON-TB Gold In-Tube test (QFT-GIT) (Qiagen) and the T-SPOT.TB test (Oxford Immunotec).22
The limitations of the century-old TST include low sensitivity, subjective interpretation of the test results, and results influenced by prior bacillus Calmette-Guérin (BCG) vaccination or infection with nontuberculous mycobacteria.23,24 Since the antigens in the IGRA test do not cross-react with BCG, it can be preferentially used as a screening test on prior foreign-born BCG-vaccinated individuals,23 which is a distinct advantage.
Older adults have a higher likelihood of false-negative TST results,25,26 possibly due to decreased delayed hypersensitivity response related to advancing age. Because IGRA is based on T-cell immune response rather than a delayed-type hypersensitivity response, it is less likely than the TST to be affected by aging.26,27 The cost differential between TST and IGRA-based QFT-GIT testing favored IGRA, since the cost per case of LTBI detection was 2.7 times more using the TST compared with QFT-GIT,28 and TST followed by QFT-GIT has been found to be cost-effective in nursing home residents with comorbidities.29
In 1990, the CDC Advisory Committee for Elimination of Tuberculosis developed recommendations for TB control in LTCFs.30 If newly admitted residents have had documented negative TST results in the previous 12 months, a single TST is needed. For residents with no prior TST results, a 2-step TST procedure should be performed during the initial evaluation. A positive TST result would warrant a follow-up chest radiograph and a clinical diagnostic evaluation.
The ATS-CDC-IDSA recommendations for LTBI treatment are largely evidence-based.31,32 However, the data supporting recommendations by specific age groups are very limited. Therapeutic drug regimens for the treatment of LTBI are summarized in Table 1. Due to the relative lack of specific data in older adults, the strength of evidence for recommendations for use in older adults is slightly modified from the ATS-CDC-IDSA guidelines.31,32 A 12-dose, once-weekly regimen of isoniazid plus rifapentine has proven to be noninferior to a 9-month, once-daily regimen (270 doses) of isoniazid monotherapy in a well-conducted large clinical trial.33 The shorter combination regimen of isoniazid plus rifapentine has proven to be more cost-effective, with a 20% higher completion rate34 and a lower risk of drug-related hepatotoxicity.33 The minimal drug-drug interactions related to rifapentine compared with rifampin makes the 12-dose rifapentine plus isoniazid combination therapy more appealing.35,36 However, older adults with HIV/AIDS on antiretroviral therapy would not be ideal candidates for the 12-dose regimen. In one study, the cost-effectiveness ratio with isoniazid for 9 months was $15,392 per 1 avoided TB case and $5225 per 1 avoided case with 3-month high-dose rifapentine plus isoniazid.37

Screening for LTBI with resultant positive TST or IGRA test results initiates a therapeutic dilemma among health care providers caring for older adults. A notable concern is that no confirmatory test exists for the diagnosis of LTBI, hence identification of LTBI cases is based on detection of host cellular immune responses. Another consideration is that the older adult population is generally frail with multiple comorbidities, and the myriad treatment options available are fraught with serious adverse effects. These considerations could explain the heterogeneity of the testing and management practices in LTCFs. Older adults are more prone to hepatotoxicity, the greatest concern when considering treatment for LTBI,38 and the risk is greater with isoniazid-containing regimens.
Current recommendations include serum liver-enzyme screening of all patients with underlying liver disease, HIV infection, and moderate to heavy alcohol consumption prior to treatment. Liver-enzyme screening must be followed by monthly testing for serial monitoring of patients with abnormal baseline values. Clinical evaluation is recommended for all patients who develop signs and symptoms of hepatotoxicity and other adverse reactions.
Discontinuation of treatment is recommended in symptomatic patients with features of hepatitis, patients with aminotransferase levels greater than or equal to 3 times the upper limit of normal, and asymptomatic patients with aminotransferase levels greater than or equal to 5 times the upper limit of normal. Pyridoxine supplementation is recommended for patients receiving isoniazid who are at increased risk of peripheral neuropathy, alcoholism, diabetes, kidney failure, malnutrition, and HIV infection.18,35
Conclusion
LTBI screening and treatment in older adults is challenging for a multitude of reasons. Although older adults have a greater incidence of comorbidities such as diabetes, underlying liver disease, immunosuppression, and co-treatment with tumor necrosis factor α inhibitors, careful consideration needs to be given to the inherent increased risk of reactivation as new short-term regimens have emerged. As older adults living in nursing homes, congregate settings, and newer innovative programs, such as PACE, face the challenges presented by infections such as TB, it is imperative to screen for and treat LTBI.
References
1. Hochberg NS, Horsburgh CR Jr. Prevention of tuberculosis in older adults in the United States: obstacles and opportunities. Clin Infect Dis. 2013;56(9):1240-1247.
2. Ricks PM, Cain KP, Oeltmann JE, Kammerer JS, Moonan PK. Estimating the burden of tuberculosis among foreign-born persons acquired prior to entering the U.S., 2005-2009. PLoS One. 2011;6(11):e27405.
3. American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161(4 pt 2):S221-S247.
4. Fortin CF, McDonald PP, Lesur O, Fülöp T Jr. Aging and neutrophils: there is still much to do. Rejuvenation Res. 2008;11(5):873-882.
5. Lönnroth K, Migliori GB, Abubakar I, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015;45(4):928-952.
6. Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5(7):e152.
7. Stead WW, Lofgren JP. Does the risk of tuberculosis increase in old age? J Infect Dis. 1983;147(5):951-955.
8. Thrupp L, Bradley S, Smith P, et al; SHEA Long-Term-Care Committee. Tuberculosis prevention and control in long-term–care facilities for older adults. Infect Control Hosp Epidemiol. 2004;25(12):1097-1108.
9. Horsburgh CR Jr, O’Donnell M, Chamblee S, et al. Revisiting rates of reactivation tuberculosis: a population-based approach. Am J Respir Crit Care Med. 2010;182(3):420-425.
10. Pratt RH, Winston CA, Kammerer JS, Armstrong LR. Tuberculosis in older adults in the United States, 1993-2008. J Am Geriatr Soc. 2011;59(5):851-857.
11. Yoshikawa TT. Tuberculosis in aging adults. J Am Geriatr Soc. 1992;40(2):178-187.
12. Pérez-Guzmán C, Vargas MH, Torres-Cruz A, Villarreal-Velarde H. Does aging modify pulmonary tuberculosis? A meta-analytical review. Chest. 1999;116(4):961-967.
13. Pang WG, Shoyinka A, McCloud M, et al. Washtenaw County Health Department Latent Tuberculosis Infection. Diagnosis and Management Protocol for HIV-Negative Adults. https://www.washtenaw.org/DocumentCenter/View/7111/WCHD-LTBI-Diagnosis-and-Management. Updated March 7, 2018. Accessed May 16, 2018.
14. American Geriatrics Society. Two-step PPD testing for nursing home patients on admission. Ann Longterm Care. 2006;14(2):38-40.
15. Finucane TE. The American Geriatrics Society statement on two-step PPD testing for nursing home patients on admission. J Am Geriatr Soc. 1988;36:77-78.
16. Prevention and control of tuberculosis in facilities providing long-term care to the elderly. Recommendations of the Advisory Committee for Elimination of Tuberculosis. MMWR Recomm Rep. 1990;39(RR-10):7-13.
17. Centers for Disease Control and Prevention. Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR. 2011;60(48):1650-1653.
18. Taylor Z, Nolan CM, Blumberg HM; American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Controlling tuberculosis in the United States: recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54(RR-12):1-81.
19. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Service Task Force. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316(9):962-969.
20. Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-γ release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and meta-analysis. J Infect Dis. 2011;204(suppl 4):S1120-S1129.
21. Sester M, Sotgiu G, Lange C, et al. Interferon-γ release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2011;37(1):100-111.
22. Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25.
23. Pai M, Zwerling A, Menzies D. Systematic review: T-cell–based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149(3):177-184.
24. Menzies D. Interpretation of repeated tuberculin tests: boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159(1):15-21.
25. Perez-Stable EJ, Flaherty D, Schecter G, Slutkin G, Hopewell PC. Conversion and reversion of tuberculin reactions in nursing home residents. Am Rev Respir Dis. 1988;137(4):801-804.
26. Woodruff CE, Chapman PT. Tuberculin sensitivity in elderly patients. Am Rev Respir Dis. 1971;104(2):261-263.
27. Diel R, Loddenkemper R, Meywald-Walter K, Gottschalk R, Nienhaus A. Comparative performance of tuberculin skin test, QuantiFERON-TB-Gold In Tube assay, and T-Spot.TB test in contact investigations for tuberculosis. Chest. 2009;135(4):1010-1018.
28. Nijhawan AE, Iroh PA, Brown LS, Winetsky D, Porsa E. Cost analysis of tuberculin skin test and the QuantiFERON-TB Gold In-tube test for tuberculosis screening in a correctional setting in Dallas, Texas, USA. BMC Infect Dis. 2016;16(1):564.
29. Kowada A. Cost-effectiveness of interferon-gamma release assays for tuberculosis screening in nursing homes. Epidemiol Infect. 2016;144(15):3215-3225.
30. Centers for Disease Control and Prevention. Prevention and control of tuberculosis in facilities providing long-term care to the elderly. MMWR Recomm Rep. 1990;39(RR-10):7-20.
31. American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52(RR-11):1-77.
32. ATS/CDC Statement Committee on Latent Tuberculosis Infection. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm Rep. 2000;49(RR-6):1-51.
33. Sterling TR, Villarino ME, Borisov AS, et al; TB Trials Consortium PREVENT TB Study Team. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365(23):2155-2166.
34. Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16(11):1269-1278.
35. Horsburgh CR Jr, Rubin EJ. Latent tuberculosis infection in the United States.
N Engl J Med. 2011;364(15):1441-1448.
36. Vernon A. Treatment of latent tuberculosis infection. Semin Respir Crit Care Med. 2013;34(1):67-86.
37. Huang Y-W, Yang S-F, Yeh Y-P, Tsao TC-Y, Tsao S-M. Impacts of 12-dose regimen for latent tuberculosis infection: treatment completion rate and cost-effectiveness in Taiwan. Medicine (Baltimore). 2016;95(34):e4126.
38. Hosford JD, von Fricken ME, Lauzardo M, et al. Hepatotoxicity from antituberculous therapy in the elderly: a systematic review. Tuberculosis (Edinb). 2015;95(2):112-122
To read more ALTC expert commentary and news, visit the homepage










