Gastric Antral Vascular Ectasia (“Watermelon Stomach”) as a Cause of Chronic Gastrointestinal Bleeding
Introduction
Gastric antral vascular ectasia (GAVE), or “watermelon stomach,” is a relatively uncommon cause of gastrointestinal (GI) blood loss. The entity is well described in the gastroenterological and endoscopic literature, but it is not as familiar to most readers of geriatric journals. The mean age of affected individuals is around 70 years, with female preponderance. It is widely believed that GAVE is underrecognized and that it is often misdiagnosed as antral gastritis. Tsai et al1 reported an average latency of five years before antral vascular ectasias are identified as the origin of GI blood loss. Therefore, it is important to keep in mind the possibility of GAVE in the differential diagnosis of chronic occult upper GI bleeding in a patient with iron deficiency anemia that is resistant to conventional treatment. We present a case of GAVE in an elderly woman and review the current literature in order to raise the level of awareness of this condition among geriatricians and others caring for older patients.
----------
The Case
Mrs. C is a 73-year old female of Iraqi-Jewish origin. She was diagnosed by her family physician as having chronic iron deficiency anemia in 2004, and was suspected of having hyperplasia with polyposis on gastroscopy in 2006. She has type 2 diabetes mellitus and chronic progressive renal failure. All of her liver tests were normal, and her history was negative for Raynaud’s syndrome or any other autoimmune disorder. Mrs. C was treated with proton pump inhibitors (PPIs) and an iron supplement. Her hemoglobin values were stable between 10 g/dL and 11 g/dL. In 2007, she was hospitalized in the department of Neurology with a right hemispheric cerebral vascular accident and left flaccid hemiplegia. There were no signs of intracranial bleeding on the initial computed tomography (CT) scan, and treatment with aspirin and low-molecular-weight heparin (LMWH) was started. Mrs. C’s admission hemoglobin level was 9.1 g/dL, the iron level was low (25 mcg/dL), and the B12 and folate levels were normal.
On the following day, the hemoglobin decreased to 8.7 g/dL, and Mrs. C received a transfusion of packed red blood cells. She was transferred to the Rehabilitation department 6 days after admission, where she was hospitalized for a total of 110 days. The rehabilitation process was slow and generally unsuccessful. The left hemiplegia did not improve, and general frailty and weakness were prominent throughout hospitalization. Mrs. C remained totally dependent on permanent nursing care. Cognitive evaluation revealed moderate dementia (Mini-Mental State Examination score of 19/30).
 The main medical problem throughout the period of hospitalization was persistent upper GI bleeding with recurrent anemia. The LMWH and then aspirin were stopped when rehabilitation was begun because of continuous blood loss. Melena was observed several times, but the blood loss was usually occult. A PPI (omeprazole) was prescribed at maximal dosage (40 mg bid), but the occult bleeding failed to respond to treatment. Mrs. C was transfusion-dependent during her stay at the Rehabilitation department, and she received a total of 18 units of packed red blood cells. Gastroduodenoscopy was performed three times; the main finding was antral erosive gastritis with no evidence of Helicobacter pylori infection.
The main medical problem throughout the period of hospitalization was persistent upper GI bleeding with recurrent anemia. The LMWH and then aspirin were stopped when rehabilitation was begun because of continuous blood loss. Melena was observed several times, but the blood loss was usually occult. A PPI (omeprazole) was prescribed at maximal dosage (40 mg bid), but the occult bleeding failed to respond to treatment. Mrs. C was transfusion-dependent during her stay at the Rehabilitation department, and she received a total of 18 units of packed red blood cells. Gastroduodenoscopy was performed three times; the main finding was antral erosive gastritis with no evidence of Helicobacter pylori infection.
Mrs. C’s not responding to standard therapy for gastric bleeding raised the suspicion that the origin of bleeding was the small intestine, so she underwent enteroscopy and a video capsule endoscopy; however, the results were noninformative. She then had a repeat gastroduodenoscopy, and this time (2.5 mo after admission to the Rehabilitation department), the gastroenterologist observed that the antral lesions were compatible with GAVE (Figure 1). The pathologic result of the antral lesion biopsy was antral mucosa with vascular ectasia (Figure 2). The joint decision with the gastroenterologists was to start endoscopic laser therapy with argon plasma coagulation (APC).
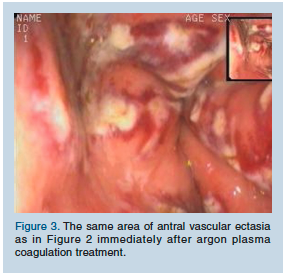
Mrs. C received three sessions of APC (40 W, Flow 1.4) on a weekly basis (Figure 3). After the third session, the hemoglobin level became stabilized at 10.5 g/dL, and blood transfusions could safely be discontinued. She was discharged home with the assistance of an aide. Two more sessions of APC were performed on an ambulatory basis (2 and 6 wk after discharge).
A hiatal hernia was diagnosed on the last endoscopy (August 2008), and the vascular ectasies were now demonstrated in both the antrum and the fundus, so APC was performed in both sites. Mrs. C’s hemoglobin levels remained stable for 4-5 months. Approximately 1 year after she had the stroke, the occult blood loss returned, and she received two more blood transfusions (February and March 2008). She was placed on iron sucrose intravenous infusions on a weekly basis, and omeprazole was switched to famotidine. At the last follow up 3 months later, Mrs. C was observed to have stayed relatively stable and had no need for further blood transfusions. APC treatment was also discontinued but remains an option should bleeding intensify.
----------
Discussion
GAVE is a relatively rare cause of chronic GI bleeding and associated iron deficiency anemia. At present, this disorder is believed to be responsible for up to 4% of nonvariceal upper GI bleeding,2 but the true rate of morbidity may be higher. GAVE can be confused with hemorrhagic gastritis or portal hypertensive gastropathy, especially if the endoscopist is not familiar with it.3 The syndrome was first described in 1953 by Rider et al4 as “erosive atrophic gastritis with marked venocapillary ectasia” in a gastrectomy specimen of an elderly woman. The unique endoscopic appearance of GAVE was described perfectly by Jabbari et al5 in 1984, who defined GAVE as “…longitudinal antral folds … converging on the pylorus, containing visible columns of tortuous red ectatic vessels.” Because its endoscopic appearance resembles the stripes on a watermelon, they labeled it “watermelon stomach.”5
 The antral involvement predominates in endoscopic presentation, but several recent reports described vascular ectasia in the proximal stomach (ie, the cardia and fundus), especially in patients with hiatal hernia.6
The antral involvement predominates in endoscopic presentation, but several recent reports described vascular ectasia in the proximal stomach (ie, the cardia and fundus), especially in patients with hiatal hernia.6
Histology is not necessary for diagnosis. Biopsies characteristically show fibromuscular hyperplasia of the lamina propria, intravascular fibrin thrombi, and an increase in the mean cross-sectional area of the lumen (ectasia) in mucosal vessels.7
The typical clinical presentation of GAVE is occult upper GI bleeding causing chronic iron deficiency anemia, and, sometimes, is transfusion-dependent. Severe acute upper GI bleeding is rare. In a classic single-center study of 45 consecutive patients with GAVE, 71% were women, and the mean age of the studied group was 73 years.8
The level of understanding of the pathophysiological changes leading to GAVE remains poor, but there are two main clinical associations thought to be causally related. One is cirrhosis of the liver, which is found in 30% of patients with GAVE: approximately 1 in 40 patients with end-stage liver disease has GAVE.9 Portal hypertension is reportedly not directly related with GAVE, but rather is influenced by the presence of liver dysfunction.10
The other association is autoimmune diseases, which are most common in patients without cirrhosis with GAVE, with incidences of 62% for autoimmune connective tissue disorders, mainly Raynaud’s phenomenon, systemic sclerosis, and sclerodactyly.11 Some patients have full-blown features of CREST syndrome (Calcinosis, Raynaud’s phenomenon, Esophageal dysmotility, Scleroderma, Telangiectasia). Definitive causal links between the development of GAVE and autoimmune diseases have not been described.1
Medical treatment for GAVE remains mainly symptomatic and includes fluid resuscitation and blood transfusions for acute bleeding episodes, iron supplementation, and, if required, repeated blood transfusions for chronic occult bleeding. There are numerous case reports that describe attempts to treat it with steroids, estrogen and progesterone, tranexamic acid, thalidomide, and alpha-interferon, but no single medical therapy has been found to stop the progression of the syndrome.1
Endoscopic laser therapy using either APC or neodymium:yttrium-aluminum-garnet (Nd:YAG) laser is the therapeutic modality of choice in actively bleeding GAVE and in cases with chronic bleeding and anemia that were unresponsive to medical therapy. Endoscopic therapies were originally based on Nd:YAG laser, but the more recent literature describes a shift towards APC.1
APC is a no-touch electrocoagulation technique that uses a high-frequency monopolar current for targeting tissues through ionized argon gas. APC treatment has several theoretical advantages, such as limited depth of penetration, which offers less risk of perforation and the symmetric spread of a coagulation effect in the surrounding target area.12
Both APC and Nd:YAG have been shown to effectively control bleeding from GAVE. Several treatment sessions may be required to abolish transfusion dependence in up to 80% of patients, and the long-term recurrence rate of chronic bleeding is high.2
Some complications of endoscopic laser treatment have been reported, among them bleeding, perforation, and the development of hyperplastic polyps.13 In addition, Probst et al14 reported that circumferential scarring of the antrum can lead to asymptomatic stenosis as late as 6 months after APC treatment.
 In the case of Mrs. C, the delay in diagnosis resembled that described in the literature1 (ie, about 4 yr from the beginning of symptomatic anemia). Her worsening of the chronic blood loss and the need for blood replacement transfusions can be well explained by her ischemic stroke that required anti-aggregant and anticoagulant treatment. Subsequent persistent blood loss precluded treatment with aspirin and LMWH, so antithrombotic medicines were withheld in spite of the risk of arterial and venous thrombotic events. Despite iron supplementation and PPIs, the patient was transfusion-dependent most of the time during hospitalization. Only the correct diagnosis of GAVE and three sessions of endoscopic APC treatment made it possible to stop the blood transfusions and to discharge the patient home. Patients are routinely given standard doses of PPIs to facilitate healing of iatrogenic ulcers and to prevent secondary bleeding, but PPIs alone have never proved their effectiveness in the treatment of GAVE. Indeed, gastric acid plays no important role in the pathogenesis of gastric vascular ectasia. Moreover, achlorhydria and secondary hypergastrinemia are speculated to promote mucosal atrophy and vascular proliferation, providing better conditions for occult gastric bleeding.
In the case of Mrs. C, the delay in diagnosis resembled that described in the literature1 (ie, about 4 yr from the beginning of symptomatic anemia). Her worsening of the chronic blood loss and the need for blood replacement transfusions can be well explained by her ischemic stroke that required anti-aggregant and anticoagulant treatment. Subsequent persistent blood loss precluded treatment with aspirin and LMWH, so antithrombotic medicines were withheld in spite of the risk of arterial and venous thrombotic events. Despite iron supplementation and PPIs, the patient was transfusion-dependent most of the time during hospitalization. Only the correct diagnosis of GAVE and three sessions of endoscopic APC treatment made it possible to stop the blood transfusions and to discharge the patient home. Patients are routinely given standard doses of PPIs to facilitate healing of iatrogenic ulcers and to prevent secondary bleeding, but PPIs alone have never proved their effectiveness in the treatment of GAVE. Indeed, gastric acid plays no important role in the pathogenesis of gastric vascular ectasia. Moreover, achlorhydria and secondary hypergastrinemia are speculated to promote mucosal atrophy and vascular proliferation, providing better conditions for occult gastric bleeding.
The recurrence rate of GAVE remains high, even after successful APC treatment. Yusoff et al15 reported a 40% recurrence rate 20 months after initial treatment. In the case of Mrs. C, the spread of vascular ectasia both in the antrum and proximal stomach was a complicating factor. Such involvement of fundus and mucosa of hiatal hernia is infrequent but well reported in the literature.6
Outcome of the Case Patient
Mrs. C had neither of the two well-known conditions associated with GAVE. As mentioned, all of her liver tests were normal, and her history was negative for Raynaud’s syndrome or any other autoimmune disorder. The other condition, less strongly associated with GAVE (chronic renal failure), was present in her case. She was transfusion-free for only 6 months after the last session of APC, but her requirement for transfused blood was much less than when she was hospitalized in the Rehabilitation department. She is receiving no anti-aggregant therapy, and she has not required any blood transfusions for the past 3 months. She is continuing with intravenous iron supplementation and H2-blockers, and her general condition is satisfactory.










