Detecting Adverse Drug Events Using a Nursing Home–Specific Trigger Tool
Adverse drug events (ADEs) are defined by the Institute of Medicine (IOM) as, “injuries resulting from a medical intervention related to a drug.”1 Institutionalized elderly experience ADEs at a rate as high as 10.8 events per 100-patient months, often as a result of polypharmacy, multiple comorbid illnesses, and difficulty with monitoring prescribed medications.2-4 This translates into approximately 135 ADEs each year in an average-size nursing home (NH; bed size of 105), or approximately 2 million events a year among all U.S. NH residents. ADEs represent the most clinically significant and costly medication-related problems in NHs, and are associated with 93,000 deaths a year and as much as $4 billion of excess healthcare expenditures.5-6 Despite the consequences and costs associated with ADEs, the vast majority of these events go undetected using traditional methods, including comprehensive chart reviews, direct observation, and voluntary reporting. Therefore, alternative surveillance strategies are needed in NHs to supplement existing detection strategies and minimize the potential consequences of ADEs.
The trigger tool methodology, developed in part by the Institute of Healthcare Improvement (IHI), greatly simplifies the chart review process by allowing rapid and systematic examination of charts to extract relevant data for the detection of potential ADEs. The technique, which requires minimal training, appears to increase the rate of ADE detection 50-fold over traditional reporting methods.7 The triggers themselves represent specific events including the ordering of certain medications (eg, antidotes such as vitamin K), the results of certain laboratory studies (eg, supratherapeutic serum medication concentrations such as digoxin level), and change in clinical status or new sign or symptom (eg, drug-induced fall or drug-related rash). Since the triggers are likely to differ based on specific clinical setting, multiple IHI trigger tools have been developed including those for mental health settings, adult inpatients, adult outpatients, adult intensive care units, adult perioperative care units, pediatric inpatients, and neonatal intensive care units.8 Many of the clinical setting-specific trigger tools have been successfully used to demonstrate the benefits of low-cost error detection strategies that produce consistent, reliable, and relevant data.9-13
Recently, a study was completed to develop a consensus list of agreed upon laboratory, pharmacy, and Minimum Data Set (MDS) 2.0 triggers to expand the use of the trigger tool methodology to the NH setting.14 The authors conducted a comprehensive literature search for potential ADE triggers, followed by an Internet-based, two-round, modified Delphi survey of physician, pharmacist, and advanced practitioner experts in geriatrics. Panelists reached consensus agreement on 40 triggers: 15 laboratory/medication combinations, 12 medication concentrations, ten antidotes, and three Resident Assessment Protocols (RAPs). Highest consensus scores (4.6; 95% CI, 4.4–4.9 or 4.4–4.8) were for: naloxone when taking opioid analgesics; phytonadione when taking warfarin; dextrose, glucagon, or liquid glucose when taking hypoglycemic agents; medication-induced hypoglycemia; supratherapeutic international normalized ratio when taking warfarin; and triggering the Falls RAP when taking certain medications.
The IHI formally adopted this set of 40 triggers as the “Nursing Home Adverse Drug Event Trigger Tool.”15 We suggest that this tool be incorporated into the consultant pharmacist medication regimen review (MRR) process. The State Operations Manual provides a definition for MRR (ie, F428) as a thorough evaluation of the medication regimen of a resident, with the goal of promoting positive outcomes and minimizing adverse consequences. The review includes preventing, identifying, reporting, and resolving medication errors or other irregularities, and collaborating with other members of the interdisciplinary team.16 According to these new guidelines, F428 emphasizes that consultant pharmacists are expected to perform MRRs at least every 30 days, and expedited reviews for short-stay residents, as well as for those residents who experience an acute change in condition.17
The IHI recommends either one of the two following strategies to detect triggers and investigate them to determine if an ADE has occurred: (1) review a sample of resident charts (letters A through I); or (2) review all resident charts (letters B through G):
A. Select a random sample of 20 resident records.
B. Obtain incident report information (eg, medication error, adverse drug event, and falls reports) from the nursing home administrator, director of nursing, or risk management (if available).
C. Review each resident record, paying particular attention to the following sections:
a. Physician orders and Medication Administration Records (MARs): Look for trigger medications.
b. Laboratory reports: Look for trigger laboratory results.
c. Consultant pharmacist medication regimen review notes, consultations, and recommendations made to the attending physician: Look for previous recommendations made for monitoring, gradual dose reduction, or to stop drug, change drug, change dose, change directions, change schedule, or other (eg, add a drug, change formulation).
d. Physician and nursing progress notes looking for acute or gradual change in condition, such as new or worsening cognitive or functional status, falls, lethargy, gastrointestinal problems, hypotension, rash, nausea/vomiting, or other adverse events that may be associated with the use of a medication. Also, take note of any unplanned hospitalization and emergency department evaluations.
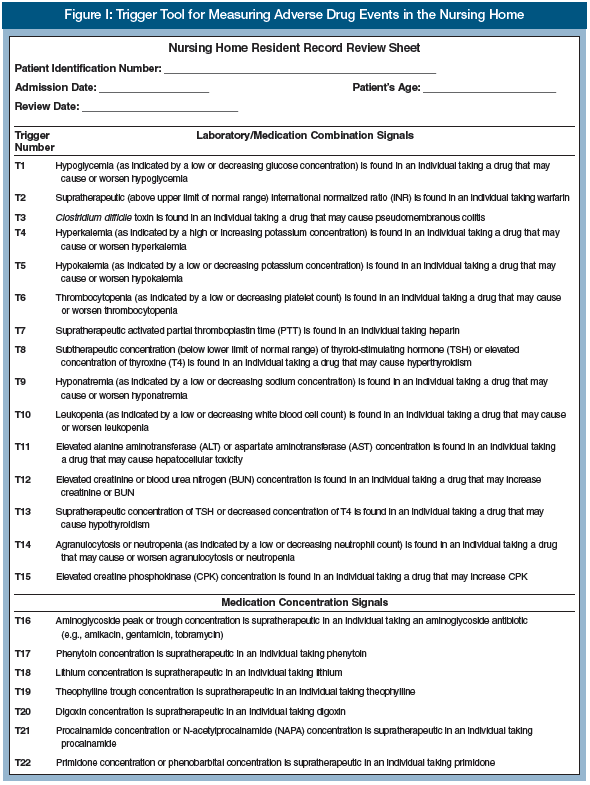
D. List all triggers found on the ADE Resident Record Review Sheet (Figure 1).
E. For each trigger found, read through the appropriate parts of the resident record to determine if an ADE has occurred. Sometimes, professional judgment will be required to make this determination. Some ADEs will result in more than one trigger; use your best judgment in determining the number of ADEs that occurred in this situation.
F. If an ADE occurred, assign a category of harm (E through I), and provide a brief description of the ADE (Figure 1).
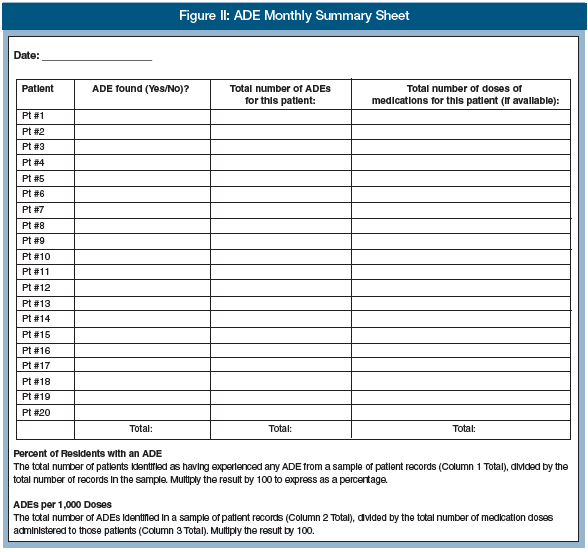
G. After you have completed the ADE Patient Record Review Sheet for the patient records in the sample, summarize your findings in the ADE Monthly Summary Sheet (Figure 2). For each patient record reviewed, document the following: whether an ADE occurred; the number of ADEs; and (if you collected data on doses) the total number of medication doses received.
H. Use the data in the ADE Monthly Summary Sheet to calculate one or both of these important measures:
a. Percent of Residents with an ADE: Defined as the total number of residents identified as having experienced any ADEs from a sample of resident records, divided by the total number of records in the sample. Multiply by 100 to express as a percentage.
b. ADEs per 1000 Doses: Defined as the total number of ADEs identified in a sample of resident records, divided by the total number of medication doses administered to those residents. Multiply the result by 1000.
I. Track the measures (Percent of Admissions with an ADE per 1000 Doses) over time in a run chart, to see if changes you are testing are making the medication system safer. You can use the Improvement Tracker on www.IHI.org to automatically track and graph these measures over time.

The IHI recommends using the results of this tool to measure the number of ADEs in a NH over time, and determine whether or not the changes a facility is making results in improvement. Similar to other NH quality improvement initiatives, the results can be summarized and reported to the quality assessment and assurance (QAA) committee that is required to meet at least quarterly, as described in F520.18 During these meetings, the committee can develop and implement plans of action to correct the future occurrence of ADEs, including monitoring the effect of implemented changes and making needed revisions to the action plans.
Although the process described above is largely manual and paper-based, the future of ADE detection in the NH setting will likely rely on utilizing health information technology. This is consistent with the IOM and other patient safety organization’s recommendation that all healthcare settings assess the safety of medication use through active monitoring systems within a culture of safety.1,16-22 Although most NHs have yet to adopt a significant amount of health information technology,23 the majority generate laboratory, pharmacy, and MDS data in an electronic format that can be used by active medication monitoring systems to automate the detection of ADEs. Recently, investigators have developed and tested an active medication monitoring system using the consensus set of NH triggers accepted by IHI.24 They found that they could detect ADEs with a high degree of accuracy and at a rate of nearly 2.5 times greater than that of usual care (ie, pharmacist-conducted manual chart review). Based on these results, the Agency for Healthcare Research and Quality (AHRQ) has recently funded a randomized controlled trial to determine the impact of this active medication monitoring system on ADE detection and management in the NH.
Acknowledgments
This study was supported by the Agency for Healthcare Research and Quality (R01HS018721), a NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (KL2 RR024154-01), National Institute of Aging grants (R01AG027017; P30AG024827), and a VA Health Services Research grant (IIR-06-062). This article is based on a book chapter, “Detecting Adverse Drug Events Using a Nursing Home Specific Trigger Tool” by Handler SM, Hanlon JT, in Geriatric Pharmaceutical Care Guidelines. 2010 ed. Covington, KY: Omnicare, Inc. The authors report no relevant financial relationships. Dr. Handler is Assistant Professor, Department of Biomedical Informatics, School of Medicine, University of Pittsburgh, Pittsburgh, PA; Division of Geriatric Medicine, Department of Medicine, University of Pittsburgh; Geriatric Research Education and Clinical Center (GRECC), VA Pittsburgh Healthcare System (VAPHS), Pittsburgh; and Center for Health Equity Research and Promotion (CHERP), VAPHS, Pittsburgh; and Dr. Hanlon is Professor, Division of Geriatric Medicine, Department of Medicine, University of Pittsburgh; Department of Pharmacy and Therapeutics, School of Pharmacy, University of Pittsburgh; GRECC, VAPHS; and CHERP, VAPHS.
References
1. Aspden P, Wolcott JA, Bootman JL, Cronenwett LR, eds. Preventing Medication Errors. Washington, DC: National Academies Press; 2007.
2. Handler SM, Wright RM, Ruby CM, Hanlon JT. Epidemiology of medication-related adverse events in nursing homes. Am J Geriatr Pharmacother 2006;4(3):264-272.
3. Gurwitz JH, Field TS, Avorn J, et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med 2000;109(2):87-94.
4.Gurwitz JH, Field TS, Judge J, et al. The incidence of adverse drug events in two large academic long-term care facilities. Am J Med 2005;118(3):251-258.
5. Bootman JL, Harrison DL, Cox E. The health care cost of drug-related morbidity and mortality in nursing facilities. Arch Intern Med 1997;157(18):2089-2096.
6. Gurwitz JH, Field TS, Rochon P, et al. Effect of computerized provider order entry with clinical decision support on adverse drug events in the long-term care setting. J Am Geriatr Soc 2008;56(12):2225-2233.
7. Cohen MR, ed. Medication Errors. Washington, DC: American Pharmacists Association; 2007.
8. Institute for Healthcare Improvement. Trigger Tool for Measuring Adverse Drug Events (IHI Tool). https://www.ihi.org/IHI/Topics/PatientSafety/MedicationSystems/Tools/Trigger+Tool+for+Measuring+Adverse+Drug+Events+(IHI+Tool).htm. Accessed March 18, 2010.
9. Resar RK, Rozich JD, Classen D. Methodology and rationale for the measurement of harm with trigger tools. Qual Saf Health Care 2003;12(suppl 2):ii39-45.
10. Rozich JD, Haraden CR, Resar RK. Adverse drug event trigger tool: A practical methodology for measuring medication related harm. Qual Saf Health Care 2003;12(3):194-200.
11. Takata GS, Mason W, Taketomo C, et al. Development, testing, and findings of a pediatric-focused trigger tool to identify medication-related harm in US children’s hospitals. Pediatrics 2008;121(4):e927-935.
12. Cohen MM, Kimmel NL, Benage MK, et al. Medication safety program reduces adverse drug events in a community hospital. Qual Saf Health Care 2005;14(3):169-174.
13. Sharek PJ, Horbar JD, Mason W, et al. Adverse events in the neonatal intensive care unit: Development, testing, and findings of an NICU-focused trigger tool to identify harm in North American NICUs. Pediatrics 2006;118(4):1332-1340.
14. Handler SM, Hanlon JT, Perera S, et al. Consensus list of signals to detect potential adverse drug reactions in nursing homes. J Am Geriatr Soc 2008;56(5):808-815. Published Online: March 21, 2008.
15. Institute for Healthcare Improvement. Trigger Tool for Measuring Adverse Drug Events in the Nursing Home. https://www.ihi.org/IHI/Topics/PatientSafety/MedicationSystems/Tools/TriggerToolforMeasuringADEsinNursing+Home.htm. Accessed March 18, 2010.
16. Martin CM, McSpadden CS. Changes in the state operations manual: Implications for consultant pharmacy practice. Consult Pharm 2006;21(12):948-961.
17. Bain KT. Adverse drug reactions and current state of drug regimen review in nursing facilities: Need for a change? Consult Pharm 2007;22(7):586-592.
18. Guidance to surveyors for long term care facilities. State Operations Provider Certification. U.S. Department of Health and Human Services and Centers for Medicare & Medicaid Services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R22SOMA.pdf. Accessed March 18, 2010.
19. National Quality Forum. Safe Practices for Better Healthcare: A Consensus Report. 2009.
20. Aspden P, Corrigan JM, Wolcott J, Erickson SM, eds. Patient Safety: Achieving a New Standard for Care. Washington, D.C.: The National Academies Press; 2004.
21. Shojania KG, Duncan BW, McDonald KM, Watchter RM. Making Health Care Safer: A Critical Analysis of Patient Safety Practices. No. 43. Rockville, MD: UCSF-Stanford Evidence-Based Practice Center, Agency for Healthcare Research and Quality; 2001.
22. Kilbridge PM, Classen DC. Automated surveillance for adverse events in hospitalized patients: Back to the future. Qual Saf Health Care 2006;15(3):148-149.
23. Alexander GL, Wakefield DS. Information technology sophistication in nursing homes. J Am Med Dir Assoc 2009;10(6):398-407. Published Online: May 29, 2007.
24. Handler SM, Hanlon JT, Perera S, et al. Assessing the performance characteristics of signals used by a clinical event monitor to detect adverse drug reactions in the nursing home. AMIA Annu Symp Proc 2008:278-282.










