The ABD’s of Long-Term Care: A Review of the Use of Some Vitamin Supplements in the LTC Setting
Nutritional supplementation is common in the long-term care (LTC) setting. Vitamin Adeficiency is rarely seen in LTC, and there is some evidence that supplementation may be harmful to these residents. Studies support benefits of vitamin B12 and folate in individuals who have a deficiency, and the relative risk of supplementation is small. Methylmalonic acid measurements and 1-mg doses of cyanocobalamin and folic acid may be prudent for deficient LTC residents. Finally, vitamin D may help to prevent a variety of conditions from infection to falls. Vitamin D may be one of the most useful single vitamin supplements that can be provided to LTC residents. How to best supplement vitamin D is not entirely clear. Daily dosing of vitamin D or intermittent dosing with megadoses (50,000 IU capsules) are both options to consider. This article reviews supplementation with vitamins A, B, and D based on recent data on risk and benefits, especially in a frail older population. (Annals of Long-Term Care: Clinical Care and Aging 2008;16[2]:28-32)
Introduction
Clinicians are often faced with concerns about adequate nutrition in older long-term care (LTC) residents. In the absence of demonstrated deficiency or clear risk, the benefit of supplementation may be debatable, but unquestionably, supplementation should not cause harm. This article addresses the supplementation of three vitamin groups: vitamin A, vitamin B complex, and vitamin D.
A vitamin deficiency is defined as “Hypovitaminosis accompanied by physiological or biochemical abnormalities.”1 Simply stated, supplementation should be used to treat or prevent the development of a deficiency. Before deciding to supplement with a vitamin, risk for deficiency and toxicity should be determined. Table I provides questions that should always be considered when contemplating vitamin supplementation.

Evaluation of risks and benefits should include cost of the supplement, administration, and monitoring for benefit and side effects. The treatment population also requires consideration. For example, vitamin A supplementation may be felt to be important for a portion of a younger population at risk for vision loss or susceptible to infection, but one could argue that such supplementation is not necessary or even appropriate for the bulk of nursing home residents. Depending on definition and measures, studies indicate that the prevalence of vitamin B12 deficiency is between 5% and 40% for subjects over age 65 years.2 Vitamin B complex has been used even in the absence of demonstrated deficiency for cognition or cardiovascular health. There is some evidence for general supplementation in the LTC setting. Vitamin D may be recommended for skeletal effects, but data indicate that nonskeletal effects may be as important in the LTC environment. Where the following three criteria are met, it may be reasonable to supplement broadly in the LTC setting with targeted assessments as clinically indicated: (1) vitamin deficiency or risk of deficiency is high; (2) risk associated with supplementation is low; and (3) evaluation of vitamin status is costly.
Vitamin A
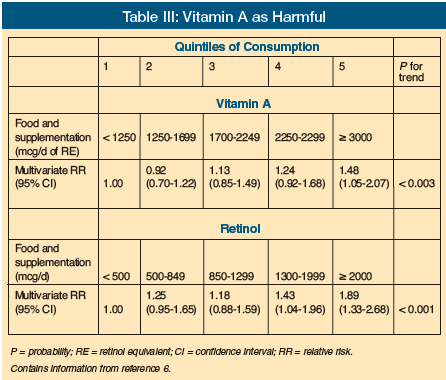
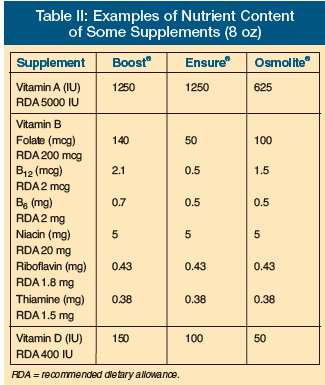 Vitamin A is fat-soluble and takes months to become depleted. Food supplements typically have amounts of vitamin A that often equate to one-quarter of the Recommended Dietary Allowance (RDA) or more. The current RDA for vitamin A is 5000 IU. Nutritional supplements typically have ample amounts of vitamin A (25% of the RDA in 8 oz portions of commonly used liquid preparations is typical; Table II). There are little data to indicate that vitamin A deficiency is a problem in adults in the United States. Even in homebound and nursing home residents followed with three-day food records, there was little evidence to support inadequate vitamin A intakes.3 The concept of vitamin A as being beneficial for infection also has not been substantiated based on a study in the LTC setting.4 Additionally, no good confirmed studies of benefit to vitamin A supplementation in the LTC setting have been published. In addition to a lack of evidence for vitamin A supplementation, there is actually potential for harm. Recent studies have looked at negative outcomes from vitamin A. Vitamin A was actually linked to reduced bone density and/or increased risk of fracture (Table III).5-7 More recently, a review of antioxidant vitamins also showed an increased risk of mortality and morbidity with excessive intake of vitamin A and beta-carotene.8
Vitamin A is fat-soluble and takes months to become depleted. Food supplements typically have amounts of vitamin A that often equate to one-quarter of the Recommended Dietary Allowance (RDA) or more. The current RDA for vitamin A is 5000 IU. Nutritional supplements typically have ample amounts of vitamin A (25% of the RDA in 8 oz portions of commonly used liquid preparations is typical; Table II). There are little data to indicate that vitamin A deficiency is a problem in adults in the United States. Even in homebound and nursing home residents followed with three-day food records, there was little evidence to support inadequate vitamin A intakes.3 The concept of vitamin A as being beneficial for infection also has not been substantiated based on a study in the LTC setting.4 Additionally, no good confirmed studies of benefit to vitamin A supplementation in the LTC setting have been published. In addition to a lack of evidence for vitamin A supplementation, there is actually potential for harm. Recent studies have looked at negative outcomes from vitamin A. Vitamin A was actually linked to reduced bone density and/or increased risk of fracture (Table III).5-7 More recently, a review of antioxidant vitamins also showed an increased risk of mortality and morbidity with excessive intake of vitamin A and beta-carotene.8
Vitamin B Complex
Some recent studies have evaluated the prevalence of vitamin B complex deficiency. While vitamins B1, B2, B3, and B6 (thiamine, riboflavin, niacin, pyridoxine) are not commonly used in the nursing home setting unless they are part of a multivitamin preparation, vitamin B12 and folate are more frequently provided in the LTC setting. Various vitamin B complex vitamins have been associated with a multitude of maladies, including deficits in cognition and bone.9-11 Such associations are commonly attributed to vitamin B12 and or folate. Occasionally, a vitamin B complex preparation is ordered when supplementation with vitamin B12 and/or folate is desired.
Despite studies showing increased sensitivity of serum or urine assays for methymalonic acid for detecting abnormalities of vitamin B12 status, most practitioners continue to measure serum vitamin B12.10-13 Studies vary in measurement of serum levels of the vitamin itself or measurement of a metabolite (eg, methylmalonic acid), but simply measuring vitamin B12 levels is no longer deemed adequate, particularly when serum B12 falls in the gray zone between 150-300 pmol/L (pg/mL). Folate and vitamin B12 have fundamental roles in central nervous system (CNS) function at all ages, especially as it relates to the methionine-synthase−mediated conversion of homocysteine to methionine, which is essential for nucleotide synthesis and genomic and nongenomic methylation, which is, relevant for cellular stability and function, including neuronal function.14
Unfortunately, the cardiovascular data have not convincingly added to the body of evidence supporting vitamin B complex supplementation.15-17 Despite a recognized link between homocysteine and cardiovascular disease, correlations between methylmalonic acid or vitamin B12 and any cardiovascular benefit have been lacking. A recent meta-analysis on folate, however, did support a role for supplementation in stroke prevention.18
The vitamin B complex, particularly vitamin B12, link appears stronger when correlated with cognition, however, although not without controversy.10,19,20 While there seems to be a correlation with vitamin B12, there remains some question with regard to folate supplementation, at least for individuals who are low at the outset.21 Nonetheless, supplementation with vitamin B12 and folate, in combination, have been linked to improvements in cognitive function.22 A recent three-year study of folate supplementation with 800 mcg per day did show significant increases in serum folate levels and reductions in homocysteine levels, as well as improvements in multiple cognitive domains, including memory.23
Many studies have demonstrated a relationship between bone density or fracture and levels of vitamin B12, folate, or homocysteine.10,24-26 While there is less controversy here, additional prospective studies of fracture reduction with vitamin B12 and folate are needed to say definitively that supplementation will produce fewer fractures.
Overall, however, when all potential benefits are taken into account along with the low risk profile, oral supplementation with vitamin B complex, especially vitamin B12 and folate, may be reasonable, though not yet recommended, even if deficiencies have not been demonstrated. Optimal oral folic acid supplementation and cyanocobalamin supplementation in older people with deficiencies appears to be 1 mg per day, about 200 times the recommended daily intake.27
Vitamin D
Recognition of the prevalence of vitamin D deficiency, especially among older adults, in combination with the benefits of supplementation make this one of the most important vitamin supplements available today.
In a 1995 study of nursing home residents who were confined indoors for at least six months, who were age 65 years or older, and who were free of diseases and medications that would interfere with vitamin D metabolism, over one-third of subjects were found to be vitamin D-deficient with 25-hydroxyvitamin D levels below 15 ng/mL, and often with evidence of secondary hyperparathyroidism.28 A decade later, vitamin D status in older persons does not seem to have improved much.29,30
Traditionally, vitamin D has been associated with bone health. Vitamin D deficiency, as defined above, refers to low 25-hydroxyvitamin D status, accompanied by either biochemical or physiological abnormalities. For example, secondary hyperparathyroidism is a well-known consequence of low vitamin D status. As such, when this biochemical aberration develops as a consequence of low vitamin D status, criteria for deficiency are met. This form of vitamin D deficiency is easy to recognize.
Since as much as 85% of 25-hydroxyvitamin D may be metabolized outside of the kidney’s conversion through alpha-hydroxylation to 1,25-dihydroxyvitamin D (the most active metabolite of vitamin D), other physiological abnormalities often result. While these other abnormalities would be sufficient to establish vitamin D deficiency, they may be more difficult to measure and, hence, one can have vitamin D deficiency even with 25-hydroxyvitamin D measures above 15 ng/mL, where laboratory measurements often reveal normal intact parathyroid hormone (PTH) measures and no other obvious biochemical abnormalities.31 Nonetheless, abnormal sway or decreased strength related to vitamin D status with normal PTH measures and 25-hydroxyvitamin D levels above 20 ng/mL would still constitute a deficient state. Conversely, some individuals may have 25-hydroxyvitamin D measurements below 20 ng/mL with no biochemical or physiologic sequelae and, thus, would be difficult to classify as deficient (although one cannot exclude ultimately greater risk for deficiency).32 The use of other terms (eg, insufficient or inadequate) are arbitrary and, physiologically and nutritionally speaking, meaningless. These terms should be avoided when describing vitamin D status.33
Low vitamin D status can result in bone loss, increased risk for fracture, abnormal balance, and reduced strength.33-35 Additionally, vitamin D deficiency can be associated with an increased risk of falls, impaired function, pain, and, perhaps, some forms of depression.36-40 Replacement with vitamin D in a vitamin D-deficient population has been shown to improve each of these parameters.34-41 It should be noted that the benefit of vitamin D to the skeleton has always been demonstrated in the presence of concomitant calcium supplementation. Recent data have also indicated the importance of vitamin D in combating tuberculosis and malignancy.42-44
The best approach to vitamin D supplementation is not clear. Because vitamin D is a fat-soluble vitamin with a relatively long half-life, daily replacement is not necessary. It may be reasonable to use an intermittent dosing strategy that employs doses far higher than the RDA of 600 IU for adults older than age 70 years. Given that older adults in the LTC setting don’t qualify as “healthy adults,” for which the RDA is designated, requirements will understandably be higher. Daily dosing should consist of at least 800 IU per day, with doses to 1600-2000 IU per day for older sunlight-deprived individuals reasonable without being concerned about toxicity. Megadose prescribing of 50,000 IU of vitamin D2 (ergocalciferol) also may be used.
Whether it is helpful to measure 25-hydroxyvitamin D levels remains unclear. Recognizing that the aforementioned doses will bring most people without diseases or medications that interfere with vitamin D metabolism into the normal range, it remains to be seen whether expensive blood testing for vitamin D status (usually more than $100 each) is cost-effective. Regardless, it will take longer to replenish a deficient individual with lower daily dosing than with megadose supplementation.
Currently, there is some debate regarding the selection between vitamin D3 (cholecalciferol) and vitamin D2 (the RDA does not distinguish between the two). While a small study of 20 people has been used to evaluate serum levels of 25-hydroxyvitamin D3 vs 25-hydroxyvitamin D2, it appears that D3 supplementation produces a more prolonged response to a 50,000 IU load, assuming that in vivo tissue levels are reflected by serum levels.45 No PTH levels were obtained in this study to reflect a physiological or biochemical efficacy difference. Most studies that have used a megadose regimen in the United States and shown clinical benefits have used vitamin D2, since vitamin D3 is not available in such doses.46
As much as 2000 IU of vitamin D3 can be administered daily without concern for overtoxicity, and some evidence indicates that doubling that amount may be safe as well.47,48 Since vitamin D status can easily be measured and the adverse event of concern is hypercalcemia, using a 2000-IU daily dose of vitamin D3 with serum monitoring of 25-hydroxyvitamin vitamin D, or calcium, as clinically indicated, is sufficient if one is concerned about toxicity. A more rapid increase in vitamin D status can be accomplished with a 50,000-IU megadose of vitamin D2. In the LTC setting it may be more desirable to use a single capsule of 50,000 I.U. once weekly or one or two 50,000-IU capsules once monthly if there is no evidence of vitamin D deficiency.
Unfortunately, these limited dosing studies of less than 50 people, in some cases using assays that would underestimate the concentration of vitamin D2, are inadequate to provide a clear approach, and more studies on supplementation with vitamin D2 and D3 are needed. In the meantime, monthly monitoring of 25-hydroxyvitamin D status along with calcium and intact PTH during the initiation of supplementation, and subsequently, based on clinical or pharmacological changes, is quite reasonable.
Patients with renal failure usually require 1,25-dihydroxyvitamin D (calcitriol) due to an inability to hydroxylate adequate amounts of this metabolite from 25-hydroxyvitamin D. Checking PTH levels as a means of monitoring the impact of supplementation may be useful in this setting.
Summary
Intake of vitamin A beyond the RDA through supplementation is not recommended for the LTC population. Supplementation with vitamin B12 and folate at 1 mg daily can be justified on a large scale in the LTC setting, though this is not currently recommended. Because vitamin D deficiency is so common and the recommendations for supplementation outlined in this article come with such low risk, vitamin D supplementation even without an initial assessment of vitamin D status in the LTC setting is reasonable.
The author reports no relevant financial relationships.
References
1. Vitamin D and calcium balance. In: National Resouce Council. Recommended Dietary Allowances. 10th ed. Washington, DC: The National Academy Press; 1989:95-96.
2. Loikas S, Koskinen P, Irjala K, et al. Vitamin B12 deficiency in the aged: A population-based study. Age Ageing 2007;36(2):177-183. Epub 2006 Dec 21.
3. Gloth FM III, Tobin JD, Smith CE, Meyer JN. Nutrient intakes in a frail homebound elderly population in the community vs a nursing home population. J Am Diet Assoc 1996;96(6):605-607.
4. Murphy S, West KP Jr, Greenough WB 3rd, et al. Impact of vitamin A supplementation on the incidence of infection in the elderly nursing home residents: A randomized controlled trial. Age Ageing 1992;21(6):435-439.
5. Melhus H, Michaelsson K, Kindmark A, et al. Excessive dietary intake of vitamin A is associated with reduced bone mineral density and increased risk for hip fracture. Ann Intern Med 1998;129(10):770-778.
6. Feskanich D, Singh V, Willett WC, Colditz GA. Vitamin A intake and hip fractures among postmenopausal women. JAMA 2002;287:47-54.
7. Michaelsson K, Lithell H, Vessby B, Melhus H. Serum retinol levels and the risk of fracture. N Engl J Med 2003;348(4):287-294.
8. Bjelakovic G, Nikolova D, Gluud LL, et al. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA 2007;297:842-857.
9. Stone KL, Bauer DC, Sellmeyer D, Cummings SR. Low serum vitamin B-12 levels are associated with increased hip bone loss in older women: A prospective study. J Clin Endocrinol Metab 2004;89(3):1217-1221.
10. Ravaglia G, Forti P, Maioli F, et al. Folate, but not homocysteine, predicts the risk of fracture in elderly persons. J Gerontol A Biol Sci Med Sci 2005;60(11):1458-1462.
11. McCracken C, Hudson P, Ellis R, McCaddon A; Medical Research Council Cognitive Function and Ageing Study. Methylmalonic acid and cognitive function in the Medical Research Council Cognitive Function and Ageing Study. Am J Clin Nutr 2006;84(6):1406-1411.
12. Clarke R, Refsum H, Birks J, et al. Screening for vitamin B-12 and folate deficiency in older persons. Am J Clin Nutr 2003;77(5):1241-1247.
13. Ho C, Kauwell GP, Bailey LB. Practitioners’ guide to meeting the vitamin B-12 recommended dietary allowance for people aged 51 years and older. J Am Diet Assoc 1999;99(6):725-727.
14. Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol 2006;5(11):949-960.
15. Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults: Scientific review. JAMA 2002;287(23):3116-3126. [Erratum in: JAMA 2002:288(14):1720.]
16. Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: A meta-analysis of randomized controlled trials. JAMA 2006;296(22):2720-2726. [Erratum in: JAMA 2007;297(9):952.]
17. Bleys J, Miller ER 3rd, Pastor-Barriuso R, et al. Vitamin-mineral supplementation and the progression of atherosclerosis: A meta-analysis of randomized controlled trials. Am J Clin Nutr 2006;84(4):880-887, 954-955.
18. Wang X, Qin X, Demirtas H, et al. Efficacy of folic acid supplementation in stroke prevention: A meta-analysis. Lancet 2007;369(9576):1876-1882.
19. Eussen SJ, de Groot LC, Joosten LW, et al. Effect of oral vitamin B-12 with or without folic acid on cognitive function in older people with mild vitamin B-12 deficiency: A randomized, placebo-controlled trial. Am J Clin Nutr 2006;84(2):361-370.
20. Balk EM, Raman G, Tatsioni A, et al. Vitamin B6, B12, and folic acid supplementation and cognitive function: A systematic review of randomized trials. Arch Intern Med 2007;167(1):21-30.
21. Malouf M, Grimley EJ, Areosa SA. Folic acid with or without vitamin B12 for cognition and dementia. Cochrane Database Syst Rev 2003;(4):CD004514.
22. Wouters-Wesseling W, Wagenaar LW, Rozendaal M, et al. Effect of an enriched drink on cognitive function in frail elderly persons. J Gerontol A Biol Sci Med Sci 2005;60(2):265-270.
23. Durga J, van Boxtel MP, Schouten EG, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: A randomised, double blind, controlled trial. Lancet 2007;369(9557):208-216.
24. Gerdhem P, Ivaska KK, Isaksson A, et al. Associations between homocysteine, bone turnover, BMD, mortality, and fracture risk in elderly women. J Bone Miner Res 2007;22(1):127-134.
25. Stone KL, Bauer DC, Sellmeyer D, Cummings SR. Low serum vitamin B-12 levels are associated with increased hip bone loss in older women: A prospective study. J Clin Endocrinol Metab 2004;89(3):1217-1221.
26. Gjesdal CG, Vollset SE, Ueland PM, et al. Plasma homocysteine, folate, and vitamin B 12 and the risk of hip fracture: The Hordaland Hhomocysteine Study. J Bone Miner Res 2007;22(5):747-756.
27. Eussen SJ, de Groot LC, Clarke R, et al. Oral cyanocobalamin supplementation in older people with vitamin B12 deficiency: A dose-finding trial. Arch Intern Med 2005;165(10):1167-1172.
28. Gloth FM III, Gundberg CM, Hollis BW, et al. Vitamin D deficiency in homebound elderly persons. JAMA 1995;274:1683-1686.
29. Moore C, Murphy MM, Keast DR, Holick MF. Vitamin D intake in the United States. J Am Diet Assoc 2004;104(6):980-983.
30. Simonelli C, Weiss TW, Morancey J, et al. Prevalence of vitamin D inadequacy in a minimal trauma fracture population. Curr Med Res Opin 2005;21(7):1069-1074.
31. Heaney, RP. Vitamin D: How much do we need, and how much is too much? Osteoporos Int 2000;11(7):553-555.
32. Binkley N, Novotny R, Krueger D, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab 2007;92(6):2130-2135. Epub 2007 Apr 10.
33. Pfeifer M, Begerow B, Minne HW, et al. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res 2000;15(6):1113-1118. [Errata in: J Bone Miner Res 2001;16(9):1735; J Bone Miner Res 2001;16(10):1935.]
34. Gloth FM III, Greenough WB 3rd. Vitamin D deficiency as a contributor to multiple forms of chronic pain. Mayo Clin Proc 2004;79:696, 699.
35. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 1992;327:1637-1642.
36. Young A, Edwards RHT, Jones DA, Benton DP. Quadriceps muscle strength and fibre size during the treatment of osteomalaocia. In: Stokes IF, ed. Mechanical Factors and Skeleton. London: Libbey;1981:137-145.
37. Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls: A meta-analysis. JAMA 2004;291:1999-2006.
38. Gloth FM III, Smith CE, Hollis BW, Tobin JD. Functional improvement with vitamin D replenishment in a cohort of frail, vitamin D deficient older people. J Am Geriatr Soc 1995;43:1269-1271.
39. Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged ≥60 y. Am J Clin Nutr 2004;80:752-758.
40. Gloth FM III, Lindsay JM, Zelesnick LB, Greenough WB 3rd. Can vitamin D deficiency produce an unusual pain syndrome? Arch Intern Med 1991;151:1662-1664.
41. Gloth FM III, Alam W, Hollis B. Vitamin D vs broad-spectrum phototherapy in the treatment of seasonal affective disorder. J Nutr Health Aging 1999;3(1):5-7.
42. Bischoff HA, Stähelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: A randomized controlled trial. J Bone Miner Res 2003;18:343-351.
43. Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006;311(5768):1770-1773. Epub 2006 Feb 23.
44. Oh K, Willett WC, Wu K, et al. Calcium and vitamin D intakes in relation to risk of distal colorectal adenoma in women. Am J Epidemiol 2007;165(10):1178-1186. Epub 2007 Mar 22.
45. Lappe JM, Travers-Gustafson D, Davies KM, et al. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am J Clin Nutr 2007;85(6):1586-1591.
46. Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 2004;89(11):5387-5391.
47. Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet 1998;351(9105):805-806.
48. Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr 2001;73(2):288-294.










