The New Era of C. difficile-Associated Diarrhea
Introduction
Clostridium difficile-associated diarrhea (CDAD) is the most common form of nosocomial diarrhea. C. difficile is a gram-positive spore-forming anaerobic bacillus that was first described in 1935, but it was not associated with antibiotic-related diarrhea and pseudomembranous colitis until 1978.1
A new era of CDAD has been entered with a significant change in nearly every aspect of the disease. With the emergence of a new more pathogenic strain, there is an increase in the number of cases and disease severity and recurrences, coupled with a decrease in response to therapy.2 Factors that have adversely affected CDAD include a change in the hospital environment to a population of more immunocompromised, elderly patients with comorbidities, associated with a reduction in nursing and housekeeping staffs and response to therapy. Additional factors include the increased use of broad-spectrum antibiotics; the increased use of acid-reducing agents such as proton pump inhibitors (PPIs); and the increased use of invasive measures, such as feeding tubes, that allow for more contamination. This has forced investigators to seek other potential treatments and to develop a multifaceted approach to managing CDAD, which includes education of hospital personnel regarding spread and prevention of CDAD, surveillance programs for detection of CDAD, institution of preventive techniques to combat CDAD, and a reduction in the use of high-risk antibiotics.
Epidemiological Features of CDAD
C. difficile is the cause of approximately 25% of all cases of antibiotic-associated diarrhea, with more than 300,000 cases per year in the United States alone. Most cases of CDAD occur in hospitals or long-term care (LTC) facilities. The incidence in the outpatient setting is rising and is at approximately 20,000 cases per year.3
_____________________________________________________________________________________________________________________________________________
Related Content
New Weapon Against C. Difficile Shows Promise
The Geography of C. Difficile Infections
_____________________________________________________________________________________________________________________________________________
Many antibiotics have been implicated in CDAD. The most commonly associated antibiotics are clindamycin, third-generation cephalosporins, and fluoroquinolones.4 Even the antibiotics used in treating CDAD, vancomycin and metronidazole, have been associated with CDAD. Therefore, clinicians should be aware that nonessential antibiotic prescriptions should be avoided since they could potentially lead to significant morbidity and mortality.5
The rate and severity of CDAD is rapidly increasing, especially among patients over age 65 years.2,3 CDAD is twice as common as all the other causes of infectious diarrhea combined.6 The rate of CDAD increased 109% and the total mortality increased to 147% from 1993 to 2003, even after adjusting for comorbidities.
In one study, CDAD community-acquired infection occurred in 22% to 28% of cases, with 43% of all patients developing symptoms at home.6 In another study, 41% of CDAD cases were diagnosed among outpatients.7 However, 14% resided in the community, and 83% were nursing home residents. An important risk factor for CDAD among the nursing home residents was recent antibiotic treatment of urinary tract infections.
Although less than 3% of all healthy adults are asymptomatic carriers, colonization of C. difficile occurs in up to 21% of hospitalized patients. Clinical symptoms of CDAD develop in only about one-third of previously asymptomatic colonized patients.8
Pathogenesis and Immunological Features of CDAD
There is a complex interaction between host factors, the environment, and an antibiotic’s effect on the gastrointestinal flora, the activity of the antibiotic against the C. difficile strain involved, local patterns of antibiotic use, and effects on toxin formation that promote CDAD. C. difficile forms spores that can persist in the environment for years. Contamination is common in hospitals and LTC facilities, especially in rooms occupied by an infected individual. Patient-to-patient transmission of the organism occurs. The organism can be cultured from many environmental surfaces in rooms and on the hands, clothing, and even the stethoscopes of healthcare workers. With the rise in use of feeding tubes to maintain nourishment of elderly, frail residents in LTC facilities, C. difficile can also be introduced by this route.
The first step in the development of C. difficile is the disruption of the normal flora, usually caused by antibiotics and anti-neoplastic or immunosuppressive drugs. Ingested spores of C. difficile survive even gastric acid, and pass through the digestive tract and germinate in the colon into the vegetative form.9 C. difficile releases toxins A and B, which are largely responsible for the disease manifestations, although there are rare cases of CDAD caused by toxin A- and toxin B-negative strains. The toxins cause cell death and release various inflammatory mediators and cytokines.10 Both toxins are necessary for the development of CDAD, which may start with the first antibiotic therapy, or six weeks or longer after antibiotic therapy is discontinued.11
Systemic antitoxin IgG and IgA and mucosal IgA all appear to be protective against C. difficile infection. Antibodies against C. difficile are present in more than 60% of adults and are associated with a lower severity of disease and risk for relapse.12 Immunization with a toxoid of toxin A has achieved active protection from clinical disease. In addition, administration of anti-toxin antibody has provided passive protection from clinical disease. Cellular immunity has not been well studied but may be of lesser importance.
A new more virulent strain of C. difficile has developed. It was first noted in an outbreak of CDAD in the Québec region of Canada in 2001 by Pepin et al.2 Although there was a four-fold increase in all patients, for persons over age 65 years the increase was ten-fold; for those older than age 80 years the increase was 20-fold. The number of persons with complicated CDAD increased seven-fold. Outbreaks caused by the same epidemic strain have been reported from many other healthcare facilities throughout Canada, the United States, and Europe.13
The severe outbreak in Canada was associated with the emergence of a new epidemic strain characterized as toxinogenic type III, pulsed-field gel electrophoresis type 1.14 It is synonymously termed BI/NAP1, or ribotype 027. The epidemic strain produced more of toxin A and toxin B, and more spores than produced by previously collected non-epidemic strains of C. difficile, thereby increasing severity of disease and environmental contamination. This new epidemic strain may be related to acquisition of resistance by the epidemic strain to fluoroquinolones associated with the increased use of this increasingly more popular class of antibiotics.2,5
As stated above, associated factors for the development and increase of the more aggressive form of CDAD affect patients in LTC facilities, including more elderly, frail immunocompromised patients with comorbidities and malnourishment. Residents who are unable to eat and are fed with feeding tubes can have spores introduced into them via this route. Nursing workloads have increased and housekeeping staffs have decreased, which has worsened the long-recognized poor compliance with washing hygiene and isolation precautions.15 There is an increased risk of CDAD with the use of PPIs and a smaller risk with H2 receptor antagonists.16 Although spores are acid-resistant, the vegetative form of C. difficile is rapidly killed by gastric acidity; spores may vegetate in the stomach in the decreased acid environment caused by the PPI agents, survive, and progress on to develop into CDAD.
Clinical Manifestations of CDAD
The clinical presentation of CDAD can vary from an asymptomatic carrier state to diarrhea and progression on to pseudomembranous colitis that may become a fulminant and life-threatening illness. Mild CDAD manifests as lower abdominal cramping pain and diarrhea without systemic symptoms. Moderate and severe CDAD presents with profuse diarrhea, abdominal distention and pain, rectal bleeding, and the systemic symptoms of fever, malaise, nausea and anorexia.8 In patients with predominantly right-sided colonic disease, there is marked leukocytosis, abdominal pain, and little or no diarrhea.
Fulminant colitis develops in approximately 3% to 8% of patients with CDAD, and appears more commonly with the newer more virulent strains of C. difficile. Fulminant colitis presents with ileus that can progress to toxic megacolon, colonic perforation, and death. Predictors of complicated CDAD include age over 65, a white blood cell count greater than 20,000 cells/mm3, and a serum creatinine greater than 2 mg/dL. Remarkably, there is often a decrease in diarrhea due to an associated severe ileus.
The epidemic of CDAD with a more virulent strain of C. difficile has been associated with an increased incidence of recurrent CDAD.5 Recurrence occurs in 20% to 30% of cases. Recurrent disease is usually noted within 5-8 days of discontinuation of treatment of CDAD, but can be delayed for weeks or even years. It is estimated that about half of the recurrences are relapses and half are new infections.17
Development and recurrence of CDAD is clearly associated with a poor serum antibody response, which would be more common in an older, more debilitated, frail elderly population with more prolonged stays in hospitals and LTC facilities.10 Also, elderly patients with comorbid illnesses continue to use antibiotics after an initial diagnosis of CDAD, which also decreases the response to treatment.18 Therefore, risk factors for recurrence include antibiotic usage, age over 65 years, a prolonged hospital stay, and the presence of comorbid illness. Recurrence by a new strain is more likely due to environmental exposure during a prolonged stay in hospitals and LTC facilities. Relapse with the same strain can occur in 20% to 30% of patients, and may be related to a lack of development of protective antibodies and re-exposure to antibiotics with persistent alterations of the protective colonic microflora.
Complications of CDAD can cause further deterioration in these patients. These include hyperpyrexia, chronic diarrhea, azotemia, renal failure, malnutrition, hypoalbuminemia, and anasarca.
(Continue to next page for Diagnosis of CDAD)
Diagnosis of CDAD (Table I)
 Nonspecific laboratory abnormalities in patients with CDAD include leukocytosis and the presence of fecal leukocytes in about 50% to 60% of cases.19 Gram staining of fecal specimens is of no value in diagnosing CDAD. The stool culture test is seldom used for clinical diagnosis since it takes 2-3 days to complete and does not distinguish toxinogenic from nontoxinogenic strains.
Nonspecific laboratory abnormalities in patients with CDAD include leukocytosis and the presence of fecal leukocytes in about 50% to 60% of cases.19 Gram staining of fecal specimens is of no value in diagnosing CDAD. The stool culture test is seldom used for clinical diagnosis since it takes 2-3 days to complete and does not distinguish toxinogenic from nontoxinogenic strains.
Although the most sensitive and specific test available for C. difficile infection is a tissue culture assay for the cytotoxicity of toxin B, it takes 1-3 days to complete and requires tissue culture facilities. The cytotoxicity assay for toxin B will detect an additional 5% to 10% of cases missed by the enzyme-linked immunosorbant assay tests (ELISA). It may be useful to perform this test if the ELISA results are negative but clinical suspicion is high.
Because of the rapidity of testing and ease of performance, ELISA for toxin A and B are now used most frequently by clinical laboratories for the diagnosis of C. difficile infection to detect either toxin A or B in the stool. These assays have a sensitivity of 71% to 94% and a specificity of 92% to 98%.20 Approximately 5% to 20% of patients may require more than one stool assay to detect toxin. Diagnostic testing during treatment, at the end of treatment, or during follow-up is not needed unless symptoms recur.
Other laboratory methods for the diagnosis of CDAD are available but are not often useful. These include the polymerase chain reaction and immunochromatographic toxin A assay.21 Radiographic imaging studies can be used to assist in the diagnosis of CDAD. Abdominal plain x-rays may reveal a dilated colon due to paralytic ileus or toxic megacolon and edematous mucosa appearing as “thumbprinting.” Abdominal computed tomography (CT) scan can reveal thickened or edematous colonic mucosa, toxic megacolon, or early perforation.
Endoscopy with either sigmoidoscopy or colonoscopy for CDAD is reserved for special situations, such as when other causes of diarrheal disease need to be ruled out, when rapid diagnosis is necessary, or when stool samples cannot be obtained because of ileus.21 The results may be normal in patients with mild disease. In more severe colitis, there may be inflammation with or without pseudomembrane formation. Although the rectum and sigmoid are involved in most cases, 10% of cases have only right-colon involvement. Due to the increased risk for intestinal perforation, endoscopy should be used cautiously as a diagnostic tool in patients with suspected CDAD.
Treatment and Management (Table II)
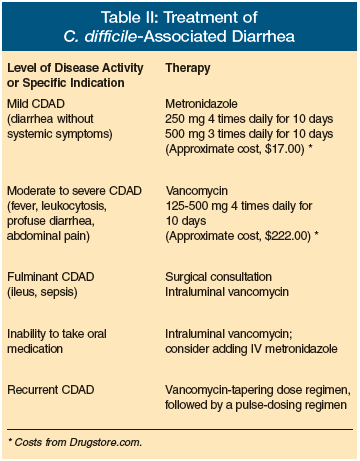 The initial treatment of CDAD includes discontinuing the inciting antibiotic therapy, if possible, and providing supportive therapy with fluids and electrolytes.22 Drugs that reduce peristalsis, such as opiates, should be avoided in patients with CDAD, because they mask symptoms and may worsen the course of disease and promote toxic megacolon. Diarrhea in CDAD will resolve without specific antimicrobial therapy in up to 25% of patients.23
The initial treatment of CDAD includes discontinuing the inciting antibiotic therapy, if possible, and providing supportive therapy with fluids and electrolytes.22 Drugs that reduce peristalsis, such as opiates, should be avoided in patients with CDAD, because they mask symptoms and may worsen the course of disease and promote toxic megacolon. Diarrhea in CDAD will resolve without specific antimicrobial therapy in up to 25% of patients.23
Antimicrobial therapy for CDAD is indicated for patients with mild to severe disease or significant underlying associated disease. Treatment of asymptomatic carriers of C. difficile provides no clinical benefit and may prolong the carrier state. 23 In severely ill patients at high risk for CDAD, antimicrobial therapy may be instituted even before the laboratory results for C. difficile are available.
Metronidazole and vancomycin hydrochloride are the antibiotics most commonly used. C. difficile is uniformly susceptible to vancomycin, but the more virulent strains of C. difficile appear to be less responsive to standard therapy. For example, older studies of the treatment of CDAD with metronidazole showed response rates of 90% to 98% and relapse rates of 5% and 7%.24 However, newer studies have reported a failure rate of 22% with a standard dose of metronidazole after 10 days of therapy and a 90-day recurrence rate of 28%.22,25 This may be explained by the fact that there is a low, variable amount of metronidazole level in the colon of CDAD patients in contrast to the very high consistent fecal levels in excess of 1000 µg/g observed with non-absorbable, orally administered vancomycin.26
Metronidazole still is considered the initial drug of choice because of concerns about the development of vancomycin-resistant enterococci (VRE) and the cost of vancomycin, which can be more than ten-fold more expensive than generic metronidazole. However, the development of complicated disease is lower in those patients initially treated with vancomycin. Changing to vancomycin with increasingly severe disease is definitely indicated. Also, the risk for the development of VRE may not be as great as was predicted in patients treated with oral vancomycin.27
In patients with mild disease with diarrhea but no constitutional symptoms, treatment with metronidazole 250 mg four times daily or 500 mg three times daily for 10 days is appropriate. In patients with moderate disease with fever, profuse diarrhea, abdominal pain, and leukocytosis, treatment with vancomycin 125 mg to 500 mg four times daily for 10 days is indicated, depending on disease severity. In patients with severe disease with dehydration, sepsis, paralytic ileus, or toxic megacolon, treatment should be intraluminal vancomycin via nasogastric tube or intracolonic infusion. In all patients who are unable to take oral medication, intraluminal vancomycin with or without intravenous metronidazole should be administered.28
Anion-binding resins such as cholestyramine and colestipol have been used to treat CDAD. They can remove up to 99% of C. difficile toxin activity. However, concerns have been raised about the use of these toxin-binding agents because they also bind to vancomycin. Thus, combination therapy with vancomycin should be used carefully with separation of resins administration and vancomycin, by giving the vancomycin either 1 hour before or 6 hours after the cholestyramine dose.
In patients with severe disease with signs of dehydration, hypotension, leukocytosis greater than 20,000 cells/mm3, elevated creatinine of 2.0 mg/dL, and sepsis, CDAD can be life-threatening. Peritonitis, paralytic ileus, and toxic megacolon are ominous signs that require surgical consultation for life-saving performance of a subtotal colectomy and ileostomy.29
Because of the increased virulence of CDAD and the decreased response to standard treatment, there is a need for newer treatments. A number of new antibiotics are being studied for the treatment of CDAD. Rifaximin, which is a broad-spectrum non-absorbable antibiotic used for prevention and treatment of traveler's diarrhea, has shown success in the treatment of C. difficile. Resistance has been rare, and cures approach those achieved with vancomycin.30 Other antibiotic agents have been investigated, including rifalazil, ramoplanin, nitazoxanide, fusidic acid, and teicoplanin.31 Tolevamer, an oral toxin-binding polymer, has also been evaluated.32
Because IgG antitoxin A has been associated with protection against development of CDAD, there has been significant interest in the use of immune-modulating agents. Intravenous immunoglobulin (IVIG) has been used in the treatment of patients with recurrent or fulminant CDAD, as well as those who have a poor response to standard therapy.33 A monoclonal antibody directed against toxin A to protect against CDAD is being studied, as well as a C. difficile toxoid vaccine.34
Techniques to restore the normal colonic flora have been attempted. Probiotics, such as Saccharomyces boulardii and Lactobacillus, have been used in an attempt to improve colonization resistance in patients with recurrent disease. However, there is insufficient evidence to prove efficacy.35 Although described as an aesthetically unpleasing treatment, stool donation instilled by a colonoscope or by nasogastric tube from healthy volunteers to restore normal colonic flora has been highly effective in some case reports.36 Another experimental approach to recurrent CDAD has been to replace the toxigenic strain with a non-toxigenic strain of C. difficile.
Treatment of recurrent CDAD has been challenging. Treatment of the initial relapse is a repeat course of the successful antibiotic used initially, most often metronidazole. For those who do not respond to metronidazole or have a second relapse, treatment with oral vancomycin is indicated. For those with multiple occurrences of CDAD, the best approach has been a combination of a tapering dose of vancomycin followed by pulse dosing, which allows time for germination of residual C. difficile spores during the delay days off antibiotics, followed by killing of the vegetative form when the vancomycin is given again. A common tapering approach is vancomycin 125 mg four times daily for 7 days, tapering to 125 mg twice daily for 7 days, then daily for 7 days. Other authors recommend starting vancomycin at a higher dose of 500 mg four times daily for 10 days, then tapering.37 After the taper has been completed, pulse-dosing regimens begin. Some authors have suggested giving vancomycin 250 mg every 2-3 days for a total of 3 weeks. Others have recommended continued lengthening of pulse interval until the vancomycin is given only once every 10 days.38
(Continue to next page for Infection Control)
Infection Control (Table III)
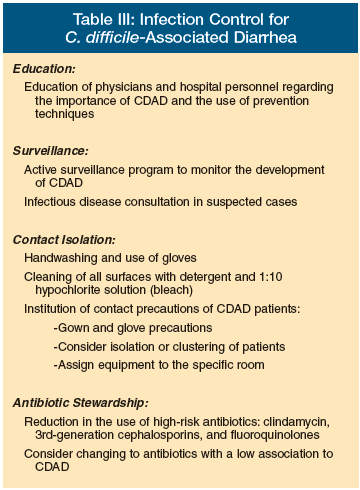 Decreasing CDAD occurrence and controlling an outbreak caused by the new virulent strain requires a multifaceted team approach that uses infection control practices to decrease patient acquisition of C. difficile from other patients, healthcare workers, and the environment and decreasing exposure to high-risk antibiotics. The increased colonization rates in hospitals and LTC facilities result from direct transfer from healthcare providers and from a contaminated environment to the patient.6 Among physicians and medical students, 75% acquired C. difficile demonstrated by positive hand cultures after patient contact. There is a direct correlation between healthcare worker hand colonization and environmental contamination.39
Decreasing CDAD occurrence and controlling an outbreak caused by the new virulent strain requires a multifaceted team approach that uses infection control practices to decrease patient acquisition of C. difficile from other patients, healthcare workers, and the environment and decreasing exposure to high-risk antibiotics. The increased colonization rates in hospitals and LTC facilities result from direct transfer from healthcare providers and from a contaminated environment to the patient.6 Among physicians and medical students, 75% acquired C. difficile demonstrated by positive hand cultures after patient contact. There is a direct correlation between healthcare worker hand colonization and environmental contamination.39
Decreasing transmission by healthcare workers will require education of physicians, LTC, and hospital personnel to increase their index of suspicion of CDAD for the early diagnosis, prevention, and treatment of CDAD. An active surveillance program of CDAD must be developed to rapidly identify trends of infection and provide an early warning system for the introduction of new strains, and to allow the facility to monitor the success of any new interventions.40 Empiric treatment of high-risk patients with diarrhea may be necessary. Early consultation with an infectious diseases specialist for additional input on infection control precautions and treatment of patients should be considered.
Decreasing environmental contamination is important, since contamination in rooms with CDAD patients can be extensive. Since the spores of C. difficile are resistant to alcohol hand gels and foams, use of vinyl gloves by medical personnel for all patients with CDAD followed by handwashing with soap and water to remove spores while still in the patient's room should be done. All surfaces should be cleaned with detergent and disinfected with unbuffered 1:10 hypochlorite solution (bleach), the only sporicidal agent available.41 The Centers for Disease Control and Prevention has recommended the use of hypochlorite-based products for disinfection of environmental surfaces in those patient care areas where surveillance and epidemiologic data indicate ongoing transmission of C. difficile.42 Other sporicidal agents are currently being studied, such as peracetic acid, hydrogen peroxide vapor, and monopercitric acid.42-45
Institution of contact precautions and isolation of patients with suspected or proven CDAD should be considered, with prompt transfer to a private room and the institution of contact isolation precautions of gloves and gowns. If private rooms are not available, which is often the case in LTC facilities, patients with CDAD should be clustered in rooms with other such patients. Consideration should be given to extend these precautions for the duration of a hospitalization in certain patients at high risk for recurrent C. difficile. Items such as stethoscopes, thermometers, and blood pressure cuffs should be individually assigned to the isolation room. Any equipment that leaves the room and is used on other patients must be adequately disinfected.
Antibiotic stewardship to reduce or limit the use and overuse of the types of antibiotics known for their high risk of CDAD, such as clindamycin, 3rd-generation cephalosporins, and fluoroquinolones, is necessary. This may require antibiotic formulary changes and restrictions. Other antibiotics with a low association with CDAD, such as piperacillin-tazobactam, should be considered as preferred antibiotics.46
Conclusion
There is a new era of CDAD, with a significant change in nearly every aspect of the disease. The emergence of a new more pathogenic strain, with an increase in the number of cases, increase in the severity of disease, and increase in the number of recurrences, along with a decrease in response to therapy, is occurring. Although traditionally considered a hospital infection, CDAD is increasingly reported in LTC facilities and in healthy persons living the community. Factors that have adversely affected CDAD include a change in the hospital environment to a population of more immunocompromised frail elderly malnourished patients with comorbidities, coupled with a reduction in nursing and housekeeping staffs and an increased use of broad-spectrum antibiotics, increased use of PPI agents, and an increased use of invasive measures such as feeding tubes.
Treatment with metronidazole and vancomycin remains the cornerstone of antibiotic treatment of CDAD. Since CDAD is increasingly less responsive to standard therapy such as metronidazole, a number of new treatments for CDAD are under investigation. Although the treatment for a first recurrence is usually a repeat course of the initial treatment, additional recurrences require a prolonged taper or pulse-dosing regimen of vancomycin.
A multifaceted approach to managing CDAD is necessary. It should include education of physicians, hospital, and LTC personnel, an active surveillance program to identify outbreaks of new resistant strains of C. difficile, preventive techniques, and restriction in the use of high-risk antibiotics.
The author reports no relevant financial relationships.
Table I: Laboratory Diagnostic Tests for C. Difficile-Associated Diarrhea Test-Tissue cytotoxic assay Advantage-Most sensitive; 94%-100% sensitivity; 100% specificity Disadvantage-Requires tissue culture facilities; 1-3 day turnaround time Test-Enzyme-linked immunosorbant assay (ELISA) Advantage-Most widely used; 80%-95% sensitivity; 92%-98% specificity; easy to use; 2-hour turnaround time Disadvantage-5%-20% of patients require more than 1 stool assay for diagnosis Test-Anaerobic culture Advantage-89%-100% sensitivity; results useful for molecular typing strains Disadvantage-Does not distinguish between nontoxin and toxin-producing Test-Polymerase chain reaction (PCR) Advantage-Very sensitive Disadvantage-Unable to distinguish asymptomatic carriage and symptomatic infection Test-Immunochromatographic toxin A Advantage-60%-85% sensitivity; simple to use Disadvantage-Does not detect A-B + toxin strains
Table II: Treatment of C. Difficile-Associated Diarrhea Level of Disease Activity or Specific Indication Mild CDAD (diarrhea without systemic symptoms) -Therapy: Metronidazole (250 mg 4 times daily for 10 days; 500 mg 3 times daily for 10 days [Approximate cost, $17.00*]) Level of Disease Activity or Specific Indication Moderate to severe CDAD (fever, leukocytosis, profuse diarrhea, abdominal pain) -Therapy: Vancomycin (125-500 mg 4 times daily for 10 days [Approximate cost, $222.00*]) Level of Disease Activity or Specific Indication Fulminant CDAD (ileus, sepsis) -Therapy: Surgical consultation; intraluminal vancomycin Level of Disease Activity or Specific Indication Inability to take oral medication -Therapy: Intraluminal vancomycin; consider adding IV metronidazole Level of Disease Activity or Specific Indication Recurrent CDAD -Therapy: Vancomycin-tapering dose regimen, followed by a pulse dosing regimen *Costs from Drugstore.com.
Table III: Infection Control for C. Difficile-Associated Diarrhea Education: Education of physicians and hospital personnel regarding the importance of CDAD and the use of prevention techniques. Surveillance: Active surveillance program to monitor the development of CDAD Infectious disease consultation in suspected cases Contact Isolation: •Handwashing and use of gloves •Cleaning of all surfaces with detergent and 1:10 hypochlorite solution (bleach) •Institution of contact precautions of CDAD patients: --Gown and glove precautions --Consider isolation or clustering of patients --Assign equipment to the specific room Antibiotic Stewardship: •Reduction in the use of high-risk antibiotics: clindamycin, 3rd-generation cephalosporins, and fluoroquinolones •Consider changing to antibiotics with a low association to CDAD









