Influenza Prevention and Treatment Strategies in the Elderly
INTRODUCTION
It has been clearly documented that influenza infections in persons over age 65 can result in serious complications leading to pneumonia, chronic disease exacerbations, hospitalization, and death.1 It is a major public health concern that American elderly persons continue to suffer from vaccine-preventable deaths, despite advancements in modern medicine and technology.2 Developed by the U.S. Department of Health and Human Services (DHHS), Healthy People 2010 is a comprehensive nationwide directive promoting health and disease prevention.3 Its primary goals are “to improve the quality and years of healthy life as well as to eliminate health disparities.”3 One of its 28 goals is to increase the number of influenza-vaccinated adults; this goal originated based on findings that only 64% of noninstitutionalized and 59% of institutionalized persons over age 65 were vaccinated for influenza in 1998.3 Healthy People 2010 aims to increase these percentages to 90% by the year 2010.4 Thus, by increasing flu vaccinations in the elderly, the objective is to decrease complications such as pneumonia, chronic disease exacerbations, hospitalization, and deaths related to influenza.
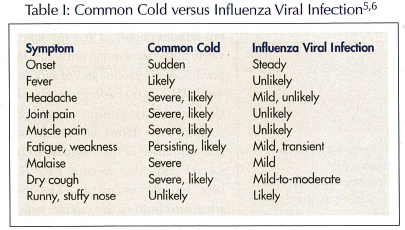
This article focuses on the seasonal influenza virus; the avian influenza A (H5N1) virus and growing risks for a pandemic flu are beyond the scope of this manuscript. As such, persons with seasonal influenza infection present with an acute respiratory illness, which is caused by the influenza virus. Table I lists its typical presentation.5,6 Among other populations, individuals over age 65 are at high risk for not only acquiring influenza but also for complications. Although most of the available morbidity and mortality data for influenza and pneumonia in the United States are limited to 1997-1998, the Centers for Disease Control and Prevention (CDC) has found that these numbers have exceeded epidemic thresholds in 2002 through 2005.7
EPIDEMIOLOGY
There are two types of influenza viruses, A and B, which can cause a respiratory infection epidemic. Generally, influenza A virus causes more severe illness than influenza B.4 The virus is composed of two types of glycoproteins on its surface, hemagglutinin and neuraminidase.4,8 The entry of the virus into the cell is aided by hemagglutinin, while neuraminidase facilitates the spread of the virus from cell to cell.9
The annual concern for influenza infections stems from the fact that new strains of the virus appear due to the variations in its genetic components: antigenic shifts or drifts. Antigenic drift arises with inefficient proofreading of the viral genome. The enzyme that replicates the viral genome, RNA polymerase, is unable to correct the mistakes that have occurred during the replication cycle. Mutations accumulate after several cycles, leading to the emergence of a new viral strain. Antigenic shift occurs when two different viruses infect a single cell and the RNA segments of both viruses are present, resulting in a new virus subtype.9
Since the influenza virus was first isolated in the 1930s, and over the past four decades, the vaccine has been used to reduce the effects of influenza in older persons.8 In 1997, influenza coupled with pneumonia was the 6th leading cause of death in the United States.5,10 When influenza-related deaths were evaluated, 90% of deaths were in individuals over age 65.5,11 In this age group, those living in long-term care facilities were at a particularly higher risk for complications of the influenza infection.5,12 This higher risk is associated with decreased immunity from advanced age, coexisting disease states, and poor lifestyle habits, such as eating and sleeping patterns. A retrospective study by Cooper13 provided some criteria for the diagnosis of severe malnutrition, which he categorized as serum albumin less than 3.4 g/dL, less than 80% of ideal body weight, and total lymphocyte count less than 1200 cells per mm3. One small study revealed that healthy, elderly patients receiving a supplement containing a modest dose of essential vitamins and trace elements showed decreased infection-related illness compared to the placebo group. Patients who had nutritional deficits at baseline showed improved immune response after taking 12 months of the modestly-dosed supplement compared to placebo.14 Given this picture, one would suspect that decreased immunity will also lead to increased susceptibility to infections. Residents in long-term care facilities would have more direct risks than elderly persons living in the community because of confinement of high-risk patients in one facility. There would be additional risks of exposure due to staff, visitors, and volunteers. These risk factors result in influenza’s impact on residents of long-term facilities, with attack rates ranging from 20-30%.5,15 It is not uncommon to find mortality rates during an influenza outbreak exceeding 5%.5,15
PREVENTION AND TREATMENT GUIDELINES
Prevention Strategies
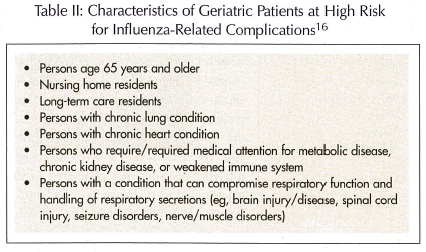
In the United States, the gold standard for the prevention of an influenza infection is immunoprophylaxis with the influenza vaccine.2,16,17 The CDC recommends annual influenza vaccination for persons who are at high risk for complications from the flu and for those individuals who either live with or care for them (ie, healthcare workers, caregivers).16,17 Table II lists characteristics specific to the elder population associated with such high risk.16 These recommendations have been extended to include patients age 50 to 64, since this population will typically have one or more medical conditions. Persons who have contraindications to the vaccine (Table III) should never receive the vaccine.16
Due to alterations of the influenza virus, the composition of the vaccine is updated annually to assure maximal efficacy.2 Protection against the virus develops approximately 2 weeks post-vaccination and persists for up to 1 year.2 The flu season in the United States may span December to March. Therefore, this vaccination should generally be administered beginning as early as October.2 Residents receiving home care or visiting nurse care should be vaccinated beginning in September.2 In an acute care setting, admitted patients should be vaccinated beginning September through March prior to discharge.2 For nursing homes and long-term care facilities, the influenza vaccination should be routinely offered and administered, with permission, to all residents during October and November, annually. Similarly, during this time, the vaccination should be offered to persons in retirement communities, at recreation centers, and in assisted living facilities.2

Two dosage formulations of the influenza vaccine are available today. The inactivated form of the vaccine, which contains killed influenza viruses, is available as an intramuscular (IM) injection; it is approved for use in the elderly.18 The IM injection is preferably administered in the deltoid muscle, which can be problematic for elderly persons due to decreased muscle mass. Therefore, an alternative injection site is the mid-lateral thigh.18 Since inactive vaccines do not affect the efficacy of either live or other inactive vaccines, simultaneous administration of the IM inactivated influenza vaccine with the IM pneumococcal vaccine and/or subcutaneous (SC) varicella-zoster vaccine in eligible elderly persons should be acceptable.2 However, concurrent administration of the varicella-zoster vaccine and other vaccines have not been formally evaluated. The live, attenuated form of the vaccine, which contains weakened influenza viruses, is available as a nasal spray.19 The nasal spray is currently approved only for healthy patients between 5 and 49 years of age, who are not pregnant.
Table IV summarizes medicinal options used for prevention and treatment of influenza infection.16,18-24

With efforts to “stretch” our vaccine supply, preliminary data from a small study suggests comparable efficacy between reduced-dose intradermal and full-dose IM administration of the influenza vaccine in elderly persons, with the exception for one strain of the virus.25 Results from a large trial show significantly higher immunogenicity for female elders receiving an IM influenza vaccine compared to a SC vaccination.26 Due to limitations of these studies, the practice of “dose-stretching” with intradermal administration and the use of subcutaneous administration are currently discouraged.
Common adverse drug reactions for the IM influenza vaccine include mild local soreness (10-64%), pain, and tenderness at the injection site.18 Not associated with the viral infection, fever, malaise, and myalgia may occur within 6-12 hours post-vaccination and persist for 1-2 days.18 Exaggerated effects of theophylline, phenytoin, and warfarin have been reported within 24-48 hours post-vaccination; however, studies have demonstrated conflicting data in support of these drug interactions. Hypersensitivity to the egg protein in the vaccine may result in a rare but severe immediate allergic reaction.
In the United States, four prescription-only antivirals are approved as treatment and/or chemoprophylactic agents: amantadine, rimantadine, oseltamivir, and zanamivir.2,20-24 These agents may be used as adjuncts, not substitutes, to the influenza vaccine. The adamantanes, amantadine and rimantadine, are antivirals with activity against influenza A only. Adamantanes, N-methyl-D-aspartate (NMDA) antagonists, work by interfering with the ion channel on the M2 protein of the influenza A virus; the M2 protein plays a vital role in viral replication. The neuraminidase inhibitors, zanamivir and oseltamivir, are antivirals that work by blocking the active site of the enzyme in both influenza A and B viruses, leading to viral aggregation and a reduction in the number of released viruses.2,20
TREATMENT STRATEGIES
As mentioned previously, the four antiviral agents also play a role in the treatment of an influenza infection. Taken within 2 days of symptoms and for 3 to 5 consecutive days, these antivirals may decrease viral transmission and duration of the illness by 1 to 2 days.
Appropriate selection of the antiviral medication is highly dependent on patient characteristics, since these agents differ in side effects, dosing, duration of therapy, age restrictions, and other factors. For instance, patients taking amantadine may experience more transient central nervous system (CNS) side effects than with rimantadine.21,22 Since these side effects are often more severe in patients who are age 65 or older, a maximum of 100 mg for either drug is recommended, especially for those who have a history of renal disease.21,22 Gastrointestinal side effects are more prominent in oseltamivir than for zanamivir.23,24 Zanamivir is contraindicated in patients with respiratory problems.24 Caution is suggested in the concurrent use of memantine with adamantanes since these agents are all NMDA antagonists.27 Concurrent use may lead to the potential risk for increased side effects. The clinical significance of this drug interaction does not appear to be consistent within the literature at this time.
To date, there are no controlled studies that compare adamantanes to neuraminidase inhibitors. However, resistance to the adamantanes is reported to be greater than to neuraminidase inhibitors. Currently circulating influenza A viruses have demonstrated clear resistance to amantadine and rimantadine.20 This cross-resistance is derived from a single mutation point at the codons for amino acids.28,29 In fact, the CDC currently does not recommend the prophylactic or treatment use of these two antiviral agents until susceptibility to these agents has been confirmed.2,28,29 With growing concerns about the emerging resistance to the neuraminidase inhibitors and the associated risks in the event of the avian influenza pandemic, prudent judgment in the use of these agents is strongly advised.
EVIDENCE-BASED MEDICINE REVIEW
The benefits from the trivalent, inactivated influenza vaccine in the elder population residing in long-term care facilities and in the community were recently investigated through a systematic literature review.8 Positive outcome of the vaccine was shown by evidence from either a reduction in laboratory-confirmed cases (ie, efficacy against influenza) or in symptomatic cases (ie, effectiveness against influenza-like illness). In the interpretation of the vaccine benefits, consideration was given to factors such as whether or not the vaccine components were well-matched to the particular viral strains, and whether viral circulation was elevated for a given season. The investigators concluded that a well-matched vaccine was 23% effective in reducing the risk of influenza-like illness in elderly residents of long-term care facilities, and was modestly effective, though not statistically significant, in community-dwellers. Contrary to the long-term care residents, the heterogeneity of the community population may have influenced the reported conclusions. Nevertheless, the well-matched vaccine is effective against complications of influenza, including hospital admission from influenza or pneumonia and all-cause mortality, regardless of residence. Two additional meta-analyses and a large-scale, 3-year study support these conclusions.30-32 Authors noted that lower vaccine efficacy may exist as the age of the patient increases.33 In general, elderly persons are more susceptible to influenza-related complications, since they tend to develop lower post-vaccination antibody titers compared to healthy adults.16 Questionable efficacy lies in the vaccine’s use as primary prophylaxis; however, many studies support the vaccine’s efficacy ranging up to 50% in preventing secondary complications, including hospitalization and death, in elderly persons.30,32,34 Through prevention of viral infections and subsequent bacterial complications, influenza vaccination may also reduce episodes of ischemic stroke (mean age = 72) and recurrent myocardial infarction (mean age = 72) in certain patient populations.35,36 The American Heart Association and American College of Cardiology recently published guidelines recommending the influenza vaccine as a secondary prevention strategy for patients with cardiovascular disease.37
A large clinical trial evaluated the immunogenicity of increasing doses of inactivated influenza vaccine administered intramuscularly to individuals age 65 years and older; study results show that doses of influenza vaccine higher then conventional dosing led to correspondingly higher serum antibody levels, frequencies of antibody responses, and putative protective titers.38
Compared to the neuraminidase inhibitors, there are more data supporting the prophylactic effectiveness of amantadine and rimantadine, since it has been used more often in the elderly population as part of many influenza outbreak-control programs.39,40 However, in one study, the prophylactic use of oseltamivir resulted in a 92% reduction in influenza illness in elderly patients in an institution.41
Individual studies evaluating the treatment efficacy of the four antiviral agents are currently limited to patients with uncomplicated influenza, and are inconclusive relative to effectiveness in patients at high risk for serious complications of influenza.16 However, clinical trials show that both zanamivir and oseltamivir decreased influenza-related illness after initiating one of the medications within 48 hours of reported symptoms in elderly persons.42,43
PRACTICAL INTERVENTION STRATEGIES
In some practice settings where immunizations are currently not provided, providers may be hesitant to provide influenza vaccination if there is no reimbursement mechanism in place for such clinics. It is important to note that Medicare is one entity that provides vaccination reimbursement. A Medicare provider number is required prior to billing. In accordance with state-specific regulations, health care professionals (eg, physicians, nurses, pharmacists) may apply for this provider number by completing a Medicare Federal Health Care Provider/Supplier Enrollment Application (CMS-885) form. Updated annually, reimbursement rates include the cost of the vaccine and administration fee. When submitting a claim, appropriate ICD-9-CM diagnosis (ie, V04.81), product, and administration codes should be used. Additional information may be found on www.medicarenhic.com.
The DHHS and Centers for Medicare & Medicaid Services (CMS) ruled on October 5, 2005, that long-term care facilities are required to annually offer each resident immunization against influenza, as well as provide education regarding the benefits and potential side effects. However, due to the autonomy and choice of each person, the vaccine cannot be forced upon individuals. It would be most prudent to clearly document in the patient’s chart when the influenza vaccine and/or antiviral chemoprophylaxis was offered but refused by the patient.
One of the challenging dilemmas of nursing home staff is detecting an influenza outbreak. This detection can be arduous, due to the fact that the symptoms can vary as well as appear similar to other respiratory ailments. Thus, each institution should define an outbreak and maintain an updated protocol for managing outbreaks at the facility.5 Two 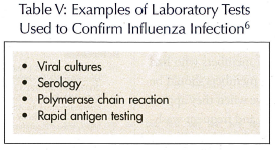 ways of accomplishing this are by initiating a surveillance strategy and yearly educational programs for residents, staff, and volunteers. The surveillance strategy should assist in early identification of infected persons or potential outbreaks through observation or influenza testing.44 The educational programs should provide a review of the institutional protocol for reducing transmission, including a review of key signs and symptoms. Usually, when three or more residents within the same nursing unit present with a temperature equal to or greater than 37.7 degrees C (100 degrees F) orally or 38.3 degrees C (101 degrees F) rectally, and symptoms consistent with influenza in a 3-day period, a clinical outbreak is declared.5,15 These symptoms, as well as results from laboratory tests (Table V), should be evaluated to confirm the outbreak.5
ways of accomplishing this are by initiating a surveillance strategy and yearly educational programs for residents, staff, and volunteers. The surveillance strategy should assist in early identification of infected persons or potential outbreaks through observation or influenza testing.44 The educational programs should provide a review of the institutional protocol for reducing transmission, including a review of key signs and symptoms. Usually, when three or more residents within the same nursing unit present with a temperature equal to or greater than 37.7 degrees C (100 degrees F) orally or 38.3 degrees C (101 degrees F) rectally, and symptoms consistent with influenza in a 3-day period, a clinical outbreak is declared.5,15 These symptoms, as well as results from laboratory tests (Table V), should be evaluated to confirm the outbreak.5
Once the outbreak is confirmed, there are several steps that long-term care health care teams can implement to reduce susceptibility and inhibit further transmission. In general, interventions may be initiated at three different levels: physician, staff, and resident. As soon as an outbreak is confirmed or suspected, a physician may want to initiate antiviral chemoprophylaxis measures using preprinted, approved physician orders to aid the swift administration of treatment and antiviral chemoprophylaxis. The chemoprophylaxis should be administered for at least 2 weeks and continuing for 1 week after the last resident case has occurred.44 To assist in containing an outbreak, staff members may once again offer the vaccine to those staff members who had previously refused the vaccine. Staff members should be strongly encouraged to take sick leave when they appear with symptoms. Face masks, gloves, and frequent washing of hands should be recommended for those ill staff members who choose to continue working.5,11 Residents with confirmed influenza or influenza-like symptoms should be isolated for 5 days after the onset of symptoms. Individuals who receive certain chemoprophylaxis (ie, amantadine and rimantadine) may also need to be isolated, since they are at risk of shedding resistant virus.5,45
Residents who had previously refused the vaccine should be offered the vaccine again. Group activities should be decentralized to separate those residents with symptoms from the residents without.5 Limitations of visitors may also be warranted.44 The facility should ensure that facial tissues, hand sanitizer and/or soap, and masks are available. These are a few initial steps in the management of outbreaks, but each institution should evaluate its own resources and staff and prepare a protocol that best suits its needs and affordability.
CONCLUSION
Serious influenza outbreaks continue to occur annually, particularly in elderly persons. With the growing resistance to antiviral agents, it is important to reserve these medications for the treatment of patients who are experiencing or who are at high risk for serious, life-threatening influenza-related illness or complications. Despite the controversy over the true effectiveness of the influenza vaccine, the vaccine is still serving its purpose for our elderly population. After all, the CDC views the goal of vaccination in the prevention of complications rather than absolute disease prevention.2,16 If doubt still remains for the true benefit of the vaccine in the elderly, a viable alternate solution would be to decrease transmission through proactive advocacy for the vaccinating of their caregivers, including healthcare professionals.
The authors report no relevant financial relationships.









