Multiple Myeloma in an Elderly Woman
CASE PRESENTATION
A 70-year-old Hispanic female presented to the emergency room with 2 weeks of persistent pain in her left hip and low back along with general fatigue. She had been involved in a motor vehicle accident 2 months earlier and had been taking a nonsteroidal anti-inflammatory medication for pain relief following the accident. She also reported a 10-pound weight loss over the last 2 months. She denied recent illnesses, falls, changes in appetite, urinary or bowel habits, or alterations in lower-extremity movements or sensation. Past medical, surgical, social, and family histories were unremarkable.
Investigation and Diagnosis
The patient’s physical examination revealed a very kyphotic elderly woman who appeared her stated age, and who was in no apparent distress. Initial laboratory work to assess her complaints of fatigue showed her to be pancytopenic: the white blood cell count was 3.8/mm3, H/H 8.1 g/dL, and 22 g/dL, respectively, and platelet count 152/cc. A complete metabolic count showed a blood urea nitrogen level of 33 and a creatinine of 2.5, with calcium at 9.9 mg/dL and total protein elevated to 9.2 g/100 mL. The albumin, liver enzyme levels, and coagulation parameters were all within normal limits.
X-rays of her extremities were significant only for diffuse osteopenia (demineralization of bone) of the forearms; no lytic or blastic lesions were identified. Pelvis and skull films were unremarkable. A radiograph of the chest revealed clear lung fields bilaterally; however, multiple new rib fractures on the right side were seen that had not been noticed on previous films from 2 months earlier.
After admission to the hospital, additional studies included blood cultures and a hepatitis panel, which were unremarkable. To further evaluate her pancytopenia, a peripheral smear was done which revealed macrocytosis and slight hypochromasia. An L-spine radiograph showed a 4-month interval progression of severe compression fractures at T12 and L1, and marked osteopenia of the spine. A magnetic resonance imaging (MRI) scan was then performed, which showed compression deformities at the vertebral bodies of T12 and L1, mild spinal canal narrowing at L4-S1, and two cystic areas at S1-S2.
Vitamin B12 was elevated at 2100 pg/mL, as was the ferritin level at 412 ng/mL. The erythrocyte sedimentation rate (ESR) was high at 155 mm/hr, and the C-reactive protein was 3.9 mg/dL; these evaluations were done to assess for ongoing inflammatory or malignant processes. A serum protein electrophoresis subsequently showed a total protein of 7.6 g/dL (normal range, 6.0 to 8.0 g/dL), and all globulin fractions were within normal limits. A 24-hour total urine protein excretion was extremely high at 7670 mg/24 hr (normal is less than 150 mg/24 hr). Urine protein was elevated to 7 g, with a positive reading of 4.6 g of kappa light chains. Serum immuno-globulin showed high immunoglobulin A (IgA) levels of 2460 mg/dL (normal, 70-300 mg/dL), with suppression of both immunoglobulin G and immunoglobulin M (IgG; IgM) levels to less than 100 mg/dL (normal IgG, 640-1430 mg/dL; normal IgM, 20-140 mg/dL). Serum kappa light chains were elevated to 4340 mg/dL, but lambda light chains were reduced. Beta-2 microglobulin was high at 13.40 mg/L. These findings were all consistent with a diagnosis of multiple myeloma.
DISCUSSION
Multiple myeloma (MM) is regarded as a clonal B-lymphocyte malignancy of the elderly that is diagnosed in approximately 15,000 people in the United States every year. Last year it was estimated that just over 11,000 of them would die from it, according to the American Cancer Society.1 It represents 1% of all cancers and 2% of all deaths related to cancer. Epidemiology of the disease shows the median age of diagnosis to be approximately age 70 years, and African Americans are affected twice as often as white Caucasians (11.3 versus 5.3 per 100,000).1 This pathologic entity is characterized by a triad of: (1) bone marrow plasmacytosis (to wit, the bone marrow is occupied by plasma cell conglomerates—called plasmacytomas or solid tumors—in concentrations greater than 10% of the total number of cells present, but can be as high as 90%); (2) multifocal lytic bone lesions occurring from hyperstimulation of osteoclastic activity; and (3) a monoclonal gammapathy that can affect the urine, serum, or both. This latter aspect is found in approximately 6% of all people age 70 years or older. The average survival rate of persons with MM after diagnosis is 3-5 years, and less than 1 year without treatment.
The causes of MM remain a mystery. It has been suggested that immunologic dysfunction and genetic factors may play a role, as well as certain chemical and radiation exposures. In the majority of cases, no clear risk factor is evident.
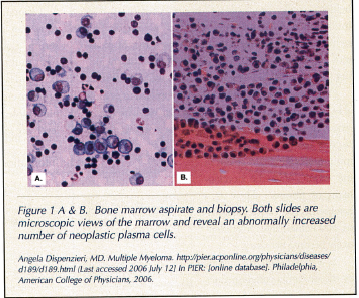
The mechanism of disease progression involves neoplastic plasma cells infiltrating the bone marrow and attaching to stromal cells via adhesion molecules. While in the marrow, these myeloma cells begin an out-of-control proliferation and overproduction of a particular line of immunoglobulins, such as IgG or IgA (Figure 1A & B).The immunoglobulins then leave the bone marrow and enter the serum. These plasma cells also produce cytokines, such as interleukin-6 (IL-6),2 which stimulate osteoclasts via an osteoclast-stimulating-factor (such as interleukin-1, tumor necrosis factor-alpha, lymphotoxin) and increase myeloma cell growth.3,4 Osteoblasts are concomitantly suppressed, promoting bone breakdown, and the immune response that would normally attack the myeloma cells is also inhibited. To support their growth, new blood vessel formation is also stimulated by the cancerous plasma cells. The disease process is locally invasive and causes lysis of bone, releasing calcium and leading to hypercalcemia.
Signs and symptoms of MM include an elevated calcium, renal insufficiency and/or failure, anemia, and bone disease.5 Bone pain and pathologic fractures may occur, as well as persistent back pain. Fatigue, neutropenia, recurrent infections, and general malaise may also be experienced by the patient.
Classification of the degree of severity of myeloma is based on the 3-stage Durie-Salmon Staging System, which takes into account hemoglobin, serum calcium, immunoglobulin and protein levels, and creatinine (Table I). An International Staging System (ISS) is also utilized, using three stages as well, differentiated based on levels of beta-2 microglobulin and albumin (Table II).

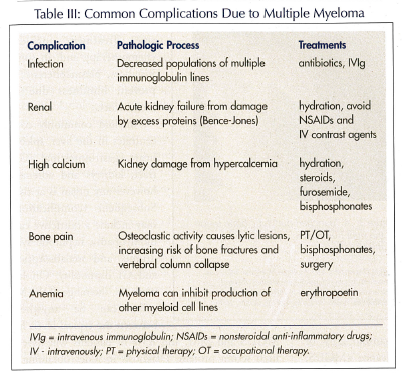
Complications of MM are many (Table III). A common problem involves the reduction in the number of immunoglobulins other than the one that is being clonally produced. Humoral activity is thus inhibited, and suppressor T-cells are activated. This sequence of events leads to an increased risk of recurrent and potentially life-threatening infections, including pneumonias, urinary tract infections, and shingles.
Progressive renal insufficiency that may lead to eventual kidney failure occurs in 20-50% of patients. This occurrence has been termed myeloma kidney or myeloma nephrosis, due to the precipitation of immunoglobulin light chains in the tubules and the development of fibrosis.6 There is a heightened risk of pyelonephritis, and hemodialysis may eventually become necessary.
As a result of encroachment by the myeloma conglomerates on myeloid precursor cells in the bone marrow, pancytopenia may occur as well. A normochromic, normocytic anemia is present in up to 60% of patients. An elevated total protein level can interfere with normal aggregation of the remaining platelets, leading to an increased risk of bleeding. Due to hyperviscosity, microinfarcts can occur in the eye and brain, causing visual and mental changes, respectively. As a result of bone destruction, increased calcium levels occur in approximately 15% of patients. The elevated concentrations of this cation in the blood can lead to weakness, altered mental status (with confusion and lethargy), depression, and constipation. A high calcium can also inhibit the effects of antidiuretic hormone in the renal tubules, leading to volume depletion and dehydration.
Multiple myeloma can contribute to the development of amyloidosis (10% of patients), osteoporosis, and may eventually transform into a leukemia.
Laboratory work-up is multifactorial and initially shows an increased total protein with an increase in serum globulin (termed hyperglobulinemia).7 Persons with multiple myeloma typically demonstrate elevated ESR and lactate dehydrogenase profiles. The tumor cells produce enormous quantities of identical immunoglobulin molecules. When quantitative immunoglobulin levels are taken using immunofixation electrophoresis, a monoclonal M-spike is typically detected, identifying an increase of a single homogeneous immunoglobulin or its fragments in serum and/or urine samples.8 It is present in approximately 70-90% of persons with MM, but not in the nonsecretory form of myeloma. If IgG is predominant, it is often greater than 5000 mg/dL; if IgA is highest, it is generally found to be in a range greater than 3000 mg/dL. These immunoglobulins are of either the kappa or lambda light chain specificity.
There is also an elevation of serum and urine free light chains, also of the kappa or lambda subtypes. Bence-Jones proteins (immunoglobulin light chains and their fragments) may appear in the urine and are nephrotoxic. However, these are present in only about 60% of cases of MM, are nonspecific, and thus may be seen in leukemias and lymphomas as well.
 Beta-2 microglobulin is a tumor marker regarded as the best single prognostic indicator of the disease. It is a component of the major histocompatibility complex Class 1 molecules involved in the immune response, as well as a normal protein in the serum.
Beta-2 microglobulin is a tumor marker regarded as the best single prognostic indicator of the disease. It is a component of the major histocompatibility complex Class 1 molecules involved in the immune response, as well as a normal protein in the serum.
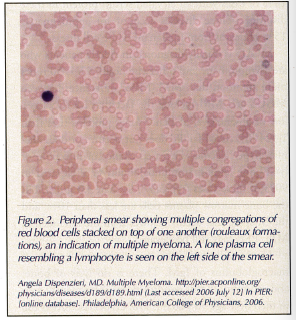
A peripheral smear can show stacked red blood cells (rouleaux formations) due to the attractive positive and negative charges between the blood cells and immunoglobulins (Figure 2).
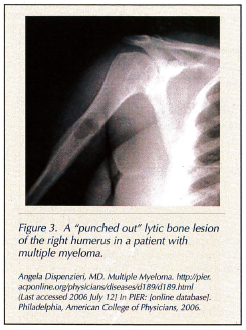
Diagnostic work-ups include a complete skeletal survey to check for lytic bone lesions, seen as “punched out” radiolucent areas (Figure 3). These lesions usually occur on the axial skeleton and skull;6 x-rays of the pelvis, spine, and long bones should be considered. A computed tomography scan may also be indicated.
A bone marrow aspirate and biopsy with plasma cell index labeling and cytogenetics will likely need to be done. If plasma cell concentrations are found to be greater than or equal to 10% of the cellular tissue in the bone marrow, the presence of MM is extremely likely. Cytogenetics may detect certain chromosomal abnormalities associated with myeloma, such as deletions or translocations on chromosomes 13, 14, and 17.9
 It should be noted that a bone scan is not helpful, since there are no osteoblastic lesions to take up the dye (as occurs in osteomyelitis, cancer spread to bone, and osteoporosis).
It should be noted that a bone scan is not helpful, since there are no osteoblastic lesions to take up the dye (as occurs in osteomyelitis, cancer spread to bone, and osteoporosis).
Multiple myeloma arises from the proliferation of a single clone of malignant antibody-producing cells.6 It is one of a number of plasma cell dyscrasias (also called paraproteinemias, dysproteinemias, and monoclonal gammopathies) that also produce elevated proteins and a M-spike, and include monoclonal gammopathy of unknown or undetermined significance (MGUS; making up approximately 66% of such cases), primary amyloidosis (8%), and Waldenstrom’s macroglobulinemia (WM; 2%). Multiple myeloma accounts for 14% of cases with an elevated serum protein in these disorders. These diseases account for approximately 15% of deaths from malignant white cell disease.8
MGUS is usually diagnosed in elderly patients who are asymptomatic and otherwise healthy. It is 50 times more common than MM. Major differences between MGUS and MM include the fact that MGUS has a low or absent level of Bence-Jones proteins (less than 1g/24 hr). Renal failure does not occur, nor does anemia, hypercalcemia, increased chance of infection, or lytic bone lesions. Immunoglobulin G is elevated in 90% of these cases (typically less than 3.0 g/dL), but the bone marrow plasma cells are less than 10%, and the M-spike that occurs is lower than the level seen in MM. ESR may still be elevated.
The M-spike in WM is due to the proliferation of macroglobulins of IgM. There is no lysis of bone, and mortality in this condition (most frequently seen in men over age 50 years) is low. Hyperviscosity is the main pathologic occurrence. The disease is slowly progressive. Treatment includes chemotherapy and, in some instances, plasmapheresis to prevent blindness due to blood clots.
Amyloid commonly concentrates in the liver, spleen, heart, gastrointestinal (GI) tract, kidneys and adrenals; however any organ is at risk. Subsequent complications may include a restrictive cardiomyopathy, nephrotic syndrome, and malabsorption and GI dismotility. On lab work-up, the range of the M-spike can be anywhere between the typical levels for MGUS and MM, and 85% of patients will have a high serum protein. Diagnosis includes obtaining a sample of tissue in which the amyloid has deposited and observing it under polarized light after applying a special stain that changes color from Congo red to green. Other areas often biopsied include a fat pad from the abdominal wall, gingivae, and rectum.
Treatment
Myeloma is considered an incurable condition. Treatment should be initiated as soon as active disease is diagnosed, and is required when infections are severe or recurrent, the level of serum paraprotein is elevated, or the bone lesions become progressive and symptomatic (ie, bone pain and fractures). A conventional treatment regimen used since 1968 has included chemotherapy with an oral alkylating agent (such as melphalan given with a high-dose glucocorticoid (ie, prednisone or dexa-methasone) for 4-7 days, for three to six cycles every 4-6 weeks (Table IV). There is a 50-60% response rate, and median survival is 2-3 years. Unfortunately, injury to stem cells can occur.
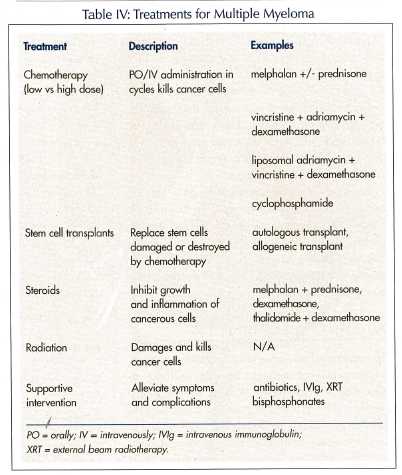
High-dose chemotherapy is more effective in destroying myeloma cells than conventional chemotherapy, but it also eliminates normal cells in the bone marrow, necessitating replacement with stem cells. Although noncurative, stem cell transplant has been found to prolong survival. Autologous stem cells are acquired from patients themselves, and in most cases peripheral blood stem cells are the ones harvested—instead of bone marrow—because hematologic recovery is faster.10
Thalidomide is a derivative of glutamic acid. It is an oral immunomodulator that possesses apoptotic and anti-angiogenic properties. It has not yet received official federal endorsement for use in MM, but approval by the Food and Drug Administration (FDA) is expected soon. Studies have shown varied responses to the use of thalidomide alone and in combination with other medications.11,12 Side effects of thalidomide are cumulative and almost always irreversible. They include fatigue, sedation, constipation, rash, peripheral neuropathy, and increased risk of deep venous thrombosis.
First-line therapy options currently include melphalan plus prednisone, vincristine/adriamycin/dexa-methasone combination, thalidomide plus dexamethasone, and a bone marrow transplant. Overall, these treatment plans have a 50-75% response rate, but all patients relapse; therefore, all the responses to treatment are transient. Survival with recurrent disease is 1-3 years. If disease relapse is refractory to treatment, death of the patient is inevitable with survival only 6-9 months.
Additional supportive measures include: hydration; bisphosphonates (such as pamidronate and zoledronic acid13 to reduce bone turnover); erythropoetin; antibiotics and intravenous immunoglobulin for infection; pain management with narcotic analgesics; and blood transfusions. Occupational and physical therapies are used to improve fatigue, and orthopedic stabilization of weight-bearing joints may need to be done, as well as the splinting of bones to secure them. Other interventions include the minimally invasive surgical procedures vertebroplasty and kyphoplasty, both of which involve the infusion of a reinforcing cement-like substance to strengthen and stabilize the diseased vertebrae. Seventy percent of patients will have improvement, but overall mortality is not affected.
The newest treatment medications for myeloma target various aspects of the MM cell simultaneously in its microenvironment in the bone marrow. These new drugs include lenalidomide and bortezomib.
Lenalidomide is an immunomodulatory drug structurally similar to thalidomide. It is also awaiting FDA approval for the treatment of MM. It is more potent than thalidomide. The mechanism of action involves cytokine modulation, stimulations of T-cell proliferation, production of interleukin-2 and interferon-gamma, and inhibition of tumor necrosis factor-alpha.14,15 Adverse effects are similar to those of thalidomide but to a lesser degree.
Bortezomib (formerly known as PS-341) is a protease inhibitor. Like lenalidomide, it has multiple effects to block myeloma cell growth. It is indicated for myeloma patients who have received at least two prior treatment regimens. Side effects involve peripheral neuropathy (usually reversible), neutropenia, and thrombocytopenia.
OUTCOME OF THE CASE PATIENT
The patient was hydrated in order to replace her depleted intravascular volume, and a thorough evaluation by hematology was conducted. A bone marrow biopsy revealed a 63% plasma cell presence, confirming the diagnosis of multiple myeloma of the IgA type. The two cystic areas seen on MRI at S1-S2 were interpreted by the radiologist as possibly representing either cysts or plasmacytomas; given other aspects of the patient’s presentation, these areas were determined to indeed represent plasmacytomas. Bence-Jones protein was found to be less than 4 g/24 hr.
The patient was designated as having Stage II (intermediate cell mass) multiple myeloma based on the Durie-Salmon Staging System, and she was started on a regimen of melphalan 200 mg/mg2 and prednisone. Simultaneous plans were initiated to perform a bone marrow transplant.
This patient had a presentation typical of multiple myeloma, with back pain and fatigue. Her symptoms can, however, be considered extremely nonspecific. In this patient’s case, the laboratory work-up was integral in making a diagnostic determination of her condition. Her skeletal survey was unusual for myeloma in that it was essentially negative. However, a patient in this age group displaying evidence of bone pain merits a differential beyond simple arthritis. Given that she had recent weight loss, a cancerous process should definitely be considered. Taken as a whole, the patient’s lab results led our investigations to the correct diagnosis.
Acknowledgments
The author would like to thank the following individuals and medical personnel whose gracious assistance, guidance, and support have made the writing of this case report possible: Marc Arel, MD, North Broward Hospital District; Wendy Hassock, RN; Cassandre LaPlanche, LPN; Turgenia Mitchell, systems analyst, North Broward Hospital District.
The author reports no relevant financial relationships.










