Psychiatric Disorders and Psychotropic Medication Use in Elderly Persons with Diabetes
INTRODUCTION
From 1980 to 2002, the number of U.S. adults with diabetes has more than doubled to 18 million, and this number is expected to double again by the year 2050. An additional 41 million U.S. adults age 40-74 years have prediabetes.1 Primary care clinicians will thus treat more persons with diabetes and their associated psychiatric disorders, and they will likely prescribe more psychotropic medications. This article focuses on psychiatric conditions relevant to diabetes: cognitive impairment, depression, and substance abuse. Up-to-date clinical data are presented on treating these psychiatric conditions and prescribing psychotropic medications in the context of diabetes management.
COGNITIVE IMPAIRMENT AND DIABETES
Epidemiology
Cognitive impairment and dementia rates are increasing due to the aging of the population and due to an increase in vascular risk factors such as diabetes. Prospective studies show that diabetes increases the risk of developing cognitive impairment. A review of 10 population-based longitudinal studies on type 2 diabetes and cognitive dysfunction confirmed an increased risk of cognitive decline and dementia from diabetes.2 Two longitudinal studies examining vascular cognitive impairment and vascular dementia showed an increased risk from diabetes.3,4 These results support diabetes as a cerebrovascular risk factor, and possibly a risk factor for Alzheimer’s disease. Overall, most studies showed a 50% increased risk of cognitive impairment and double the risk of dementia.2,5,6 This association is stronger in persons who have had diabetes longer and in those who take insulin.7
Health Outcomes
Cognitive impairment in persons with diabetes is related to functional impairment, poorer self-care, and higher health and social service use. In two studies of older adults with diabetes in rural areas, those with lower cognitive skills needed more assistance with diabetic self-care monitoring and personal care, were less likely to attend a specialist diabetes clinic, and were more likely to have been hospitalized in the past year.8,9 Patients and their caregivers considered diabetes a low priority, in spite of A1C levels averaging 10.9%.
In addition to poor self-care and greater health service use, mortality is also high in this group. In a population-based study of the elderly, adjusted 6-year relative risks of mortality in those with cognitive impairment and in those with diabetes were similar: 1.68 (1.53-1.86) and 1.62 (1.44-1.83), respectively. While no interaction was found between cognitive impairment and diabetes on risk of mortality, the proportion surviving with both illnesses after 6 years was approximately 50%.1
Mechanisms
Epidemiologic evidence supports the role of diabetes as a vascular risk factor for vascular cognitive impairment and vascular dementia. In addition, diabetes and Alzheimer’s disease have a unique relationship thought to be related in part to the ApoE4 allele, which is a known risk factor for development of Alzheimer’s disease. An additionally increased risk of Alzheimer’s disease has been found in individuals with the ApoE4 allele who have diabetes compared to those with ApoE4 and no diabetes.11 Although diabetes does not independently produce any of the usual brain pathology associated with Alzheimer’s disease, one study showed that diabetes dramatically increased the amyloid deposition and neurofibrillary tangles in people with the ApoE4 genotype compared to those without the ApoE4 genotype.12 Therefore, in people with the ApoE4 allele, diabetes may lead to a more dramatic increase in Alzheimer’s disease pathology.
Cognitive Deficits on Clinical Examination
Older adults with type 2 diabetes score more poorly than those without diabetes on multiple neuropsychological tests. Women with even prediabetes, or impaired fasting glucose, perform more poorly on cognitive tests than women with normal blood glucose levels.13 A more definitive result than these cross-sectional study results was that of the Framingham prospective study, which showed an independent effect of diabetes on the development of impaired verbal memory and abstract reasoning.7 Other large, cross-sectional studies that assessed cognitive domains found impaired attention and verbal fluency in persons with diabetes as compared to those without diabetes.14 A recent study of a group of older adults without dementia with and without insulin resistance found worse executive function in the insulin-resistant group.15 These results and the results of the prospective studies on diabetes and dementia suggest that the cognitive changes may occur early in the development of diabetes, and that the cognitive deficits are mostly consistent with frontal-subcortical deficits.14
Some studies suggest that deficits in cognitive function are associated with poorer glycemic control. Other studies suggest that factors such as depression, cardiovascular disease, and cerebrovascular disease may increase these deficits. Early onset of type 2 diabetes, poor glycemic control, and the presence of micro- and macrovascular disease may interact to produce early cognitive deficits. In older adults, diabetes likely interacts with other dementing processes, such as vascular disease and Alzheimer’s disease, to hasten cognitive decline.16
Acute Effects of Elevated or Reduced Blood Glucose Level
A linear relationship exists between glucose uptake in the brain and arterial glucose concentration. Hypoglycemia produces neuroglycopenia (cognitive-related) and autonomic changes, which occur at 3.0 mmol glucose/liter17 (54 mg/dL), while deficits in all areas occur at 2.2 mmol glucose/liter (40 mg/dL).18
Speed-dependent and attention tasks are most impaired while accuracy is preserved. At 2.2 mmol glucose/liter, multiple cognitive domains become impaired. Cognitive function may take 40-90 minutes to recover after blood glucose has normalized. In addition, patients are drowsy, uncoordinated and have speech difficulty. The patient experiences autonomic changes such as sweating, tremor, and heart palpitations. The hypoglycemic episode can also bring on psychiatric symptoms such as anxiety, depressed mood, paranoia, and fear of future hypoglycemic episodes. Recurrent severe hypoglycemic episodes can have long-term effects on cognition.
During hyperglycemia, cognitive changes have not been shown to occur until levels are beyond 382 mg/dL.
In summary, persons with type 2 diabetes have an increased risk of developing irreversible cognitive impairment and dementia, more typically of a vascular type. Finally, persons with diabetes are at risk of reversible cognitive impairment from hypoglycemia, as well as irreversible cognitive deficits from frequent, severe hypoglycemic episodes.
Treatment and Prevention of Cognitive Impairment in Diabetes
No well-controlled studies have confirmed that improved diabetes treatment benefits cognitive outcome.19 Potential treatments include the following:
• Correct metabolic disorders
• Maintain optimal glycemic control
• Treat depression
• Treat cardiovascular and cerebrovascular risk factors
• Provide caregiver support for diabetes self-care activities (diet, exercise, pill/insulin-taking, blood-glucose checks)
Pharmacologic Treatments
Specific pharmacologic treatments with cholinesterase inhibitor medication or memantine can delay cognitive decline in those with Alzheimer’s disease, and probably those with vascular dementia20 (Table.) However, studies in long-term care facilities lack sufficient quality to guide pharmacologic decision making.21
The dose of memantine, an NMDA-receptor antagonist for moderate-to-severe Alzheimer’s disease, requires reduction in moderate and severe renal failure, a common condition in diabetes, since its major route of elimination is renal. Galantamine, a cholinesterase inhibitor, also requires dose reduction in renal insufficiency (Table.)
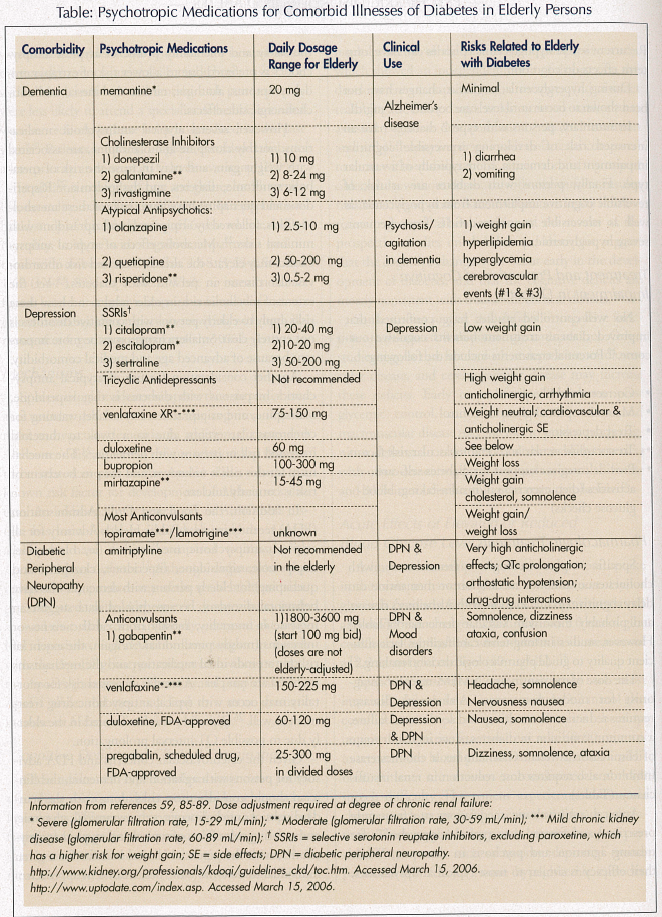
Atypical antipsychotic medications are frequently prescribed and favored over typical antipsychotics for treating agitation and psychosis in dementia. While their efficacy is similar to most typical antipsychotics, the safety and tolerability profile of atypical antipsychotics is preferred due to a lower risk of extrapyramidal symptoms, akathisia, tardive dyskinesia, and anticholinergic side effects.
However, several atypical antipsychotic medications, notably clozapine and olanzapine, are associated with weight gain, and may increase the risk of metabolic syndrome, diabetes, and dyslipidemia.22 Risperidone and quetiapine have lower risks of these metabolic effects, followed by aripiprazole and ziprasidone with minimal risks.22 Metabolic effects of atypical antipsychotics may elevate the already increased risk of cardiovascular disease in persons with diabetes.23 Yet the extent of metabolic risk in older adults and how these risks apply to elderly persons with cognitive disorders is not entirely clear. Smaller changes may be more important because of advanced age and medical comorbidity.
Another consideration for use of atypical antipsychotics in persons with diabetes is that risperidone, olanzapine, and aripiprazole carry a label warning for cerebrovascular events, due to a two- to threefold increased risk in persons with dementia.24 The mechanism for this risk is unknown, and the precise extent of risk is currently unclear.
In addition, the Food and Drug Administration (FDA) recently issued a Public Health Advisory for all atypical antipsychotic medications (ie, olanzapine, aripiprazole, ziprasidone, risperidone, clozapine, and quetiapine) for elderly persons with dementia who have behavioral disorders, because pooled data suggest an increase in mortality, mostly from cardiac events or infections (mostly pneumonia).25 Again, the extent of risk for an individual medication and the mechanisms involved are not clear. A similar increased risk for mortality may occur with typical antipsychotic drug treatment as well.26 Ziprasidone is less favored in the elderly due to possible QT-interval prolongation.
Given the cerebrovascular concerns and FDA advisory for persons with agitation and dementia, the clinician should consider these risks along with the standard risks of anticholinergic, extrapyramidal, glycemic, and sedating side effects of typical and atypical antipsychotics, with the potential clinical benefits for a given individual to determine the best treatment approach.
DEPRESSION AND DIABETES
Epidemiology
Major depression affects about 10% of older adults in the primary care setting, but nearly 18% of those with type 2 diabetes. Adults with diabetes have twice the rate of major depression compared to those without diabetes (odds ratio [OR] = 2.0, 95% confidence interval [CI] = 1.8-2.2).27-29 Rates for type 1 and type 2 diabetes are similarly increased.30
Depression and depressive symptoms constitute a risk factor for developing type 2 diabetes, and in fact may accelerate the onset of diabetes complications.31 Multiple longitudinal studies have found that depression remains a significant risk factor for development of diabetes, even after controlling for potential confounding factors such as age, race, gender, socioeconomic status, education, use of health services, other psychiatric disorders, and body weight.31-34
One prospective study looked at the possible causal relationship of depression predicting diabetes, and the reverse, diabetes predicting depression. Similar to previous studies, results showed that depressed mood was a risk factor for type 2 diabetes in older adults; however, there was no significant relationship in the direction of diabetes predicting depression.34 Yet an increased prevalence of depression generally occurs in patients who suffer complications of their diabetes.35 In a meta-analysis of 27 studies, depression was significantly associated with a variety of diabetes complications (diabetic retinopathy, nephropathy, neuropathy, macrovascular complications, and sexual dysfunction).35 Of the various diabetes complications, symptomatic neuropathy was most strongly associated with depression and minor mental disorder in one study.36
The more severe the diabetes complications, the more severe are the depressive symptoms, according to a 12-month descriptive study.36 While all patients are at risk for depression, those with diabetes complications may be a particularly high-risk group for depression.
Health Outcomes
Both diabetes and depression are associated with numerous poor outcomes that affect diabetes management, diabetes control, quality of life, and health care costs.37 Specifically, over 75% of adults with diabetes and depression are functionally impaired, while 50% are impaired with either disease present; 25% without either disease are functionally impaired.38 Adults with diabetes and depression have poorer compliance with their self-care activities (eg, diet, physical activity, medication adherence, blood glucose self-monitoring).39,37 They also have poorer glycemic control and increased micro- and macrovascular complications.35,40 Mean health care expenditures for persons with diabetes and depression was $247 million in 1996 compared with $55 million for persons with diabetes without depression (P < 0.0001).41
Mechanisms
Diabetes and depression may be linked by increased counter-regulatory hormone release by catecholamines, glucocorticoids, growth hormone, and glucagons. These hormones may impair glucose tolerance and increase blood glucose via sympathoadrenal activation, hypothalamic-pituitary-adrenocortical hyperactivitiy, and alterations in the activity of the hypothalamic-growth hormone axis.42 Hypothalamic-pituitary-adrenal axis dysregulation may affect mood and result in increased release of cortisol, decreased glucose uptake, and elevated glucose levels.43
The mechanism for the higher prevalence of depression in persons with diabetes with complications is unclear. Cerebrovascular changes in frontal-subcortical regions may account for depression or worsening depressive symptoms44 by damaging key mood regulatory regions.45 For instance, white matter hyperintensities and basal ganglia lesions have been shown to be associated with depression.46 Depression may also be related to the psychosocial conditions and circumstances surrounding diabetes in some patients, such as poverty, lack of social support and more complications, perceived disability, and worse adherence.47,48
Depression Treatment Considerations in Diabetes
Depression is recognized and treated in fewer than 25% of persons with diabetes.49 All three modalities—psychotherapy, antidepressant treatment, and enhanced care management—treated depression effectively in persons with diabetes, and in some studies, showed better glycemic control.50
Nonpharmacologic Treatment
Cognitive behavioral therapy (CBT) improves depression in diabetes; depression remitted in 85% of those receiving CBT as compared with 27% of those not receiving CBT in one study (P < 0.001).51 Enhanced care management for depression also showed improved mood, functioning, and exercise.52
Pharmacologic Treatments
Antidepressant medications. The selective serotonin reuptake inhibitors (SSRIs) are considered the safest of antidepressants in older persons.53 Sertraline demonstrated effectiveness in persons with type 2 diabetes with depression in a small, open, single-blinded trial using a 50-mg daily dose,54 and in a trial of elderly subjects randomized to sertraline (50-100 mg) and fluoxetine.55 In a randomized, placebo-controlled trial, fluoxetine treatment at 40mg per day led to improvement in depression and a reduction in hemoglobin A1C levels.50 Expert consensus guidelines on depression treatment for older persons gave the highest ratings for efficacy and tolerability to citalopram and sertraline.53
Many antidepressant medications increase the risk of appetite and weight gain, which may exacerbate type 2 diabetes. Tricyclic antidepressants (TCAs), in particular, amitriptyline, may induce 1-3 pounds weight gain per month. The nonselective monoamine oxidase inhibitors (MAOIs), which are rarely used in the elderly due to food restrictions and medication interactions, possess a weight gain risk as well.
The SSRIs have demonstrated a lower weight gain risk. In a comparative, 12-week study of fluoxetine and sertraline in elderly subjects, the average weight loss with fluoxetine was 2.8 pounds, and with sertraline was 0.6 pounds.55 In a Cochrane meta-analysis of obese persons with type 2 diabetes, average weight loss with fluoxetine was 3.4 kg at 8-12 weeks and 5.8 kg at 52 weeks.56 Use of citalopram and escitalopram have both demonstrated mild weight gain of 1.0 kg and 0.4 kg, respectively, over a 24-week trial in primary care patients.57 In a trial of citalopram in elderly subjects, no clinically significant weight gain was observed.58 Paroxetine may be more prone to induce weight gain among the SSRIs.59
Among novel antidepressants, mirtazapine has a high potential for weight gain in younger adults, so it may not be an ideal choice for many persons with diabetes. Specific data on the use of mirtazapine in elderly persons with diabetes, however, is lacking. Mirtazapine interferes with a membrane protein called GLUT4, which transports glucose into target cells across the plasma membrane.58 This may be a relevant mechanism for the increased incidence of weight gain associated with this medication, and may have additional implications for persons with diabetes. Venlafaxine has shown no clinically significant weight gain in the elderly,59 and bupropion and bupropion SR are associated with a dose-related weight loss.60
In selecting an antidepressant with less likelihood of substantial weight gain, sertraline, fluoxetine, citalopram, escitalopram, venlafaxine, and buproprion present favorable options for persons with type 2 diabetes and depression.
Mood stabilizer medications. Anticonvulsants and lithium have been used successfully to treat bipolar affective disorder (BAD) type I and II and other related psychiatric conditions.
Lithium. Lithium is infrequently used in elderly persons, and when used, it is reserved for bipolar disorder, due to its very narrow therapeutic window in the elderly. Weight gain can range between 5 kg and 15.6 kg (nearly 35 lb) in 2 years,61 and lithium clearance is highly dependent on renal function.
Anticonvulsants. Valproate can induce a low resting metabolic rate and substantial weight gain that does not taper off. It may be associated with endocrine dysfunction, including insulin resistance and hyperinsulinemia.62 Carbamazepine can produce weight gain that is less than that of valproate. Lamotrigine and topiramate are two anticonvulsants used in BAD that can promote weight loss. These bipolar medications have not been tested specifically in the elderly population with diabetes. Thus, careful consideration of these agents is needed in older persons with diabetes and mood disorders in order to optimize mood stabilization while minimizing potential adverse effects on diabetes.
ALCOHOL AND DIABETES
Epidemiology
Excessive alcohol intake increases the risk of developing diabetes. Four drinks or more per day can increase the risk by 43%.63 The mechanism that links heavy alcohol drinking to diabetes is unknown; however, recurrent alcoholic pancreatitis may contribute by causing the destruction of alpha and beta cells in the pancreas. These individuals may go on to develop brittle diabetes. In contrast, light-to-moderate alcohol consumption (one to three drinks per day) lowers the risk of diabetes by one-third to one-half,63 possibly by increasing insulin sensitivity.64
Clinical Findings
A potentially acute adverse effect of alcohol intake is that it affects blood sugar levels, which can result in hypoglycemia or hyperglycemia in persons with diabetes. Alcohol inhibits gluconeogenesis as well as glycogenolysis, two major pathways by which the body makes glucose. Drinking alcohol without food or in a poorly nourished state can lead to dangerously low blood sugar levels.64,65 In contrast, well-nourished persons with diabetes can develop significant hyperglycemia from chronic alcohol intake.
A favorable effect of alcohol in persons with diabetes is that light-to-moderate intake lowers the incidence of coronary heart disease. This may be related to a reduction in markers of inflammation and endothelial dysfunction, or to increased high-density lipoproteins.66 Overall, persons with diabetes require additional glycemic attention from their providers if they drink alcohol.
Treatment
Interventions in the adult primary care setting can be successful in reducing heavy drinking, according to a number of studies.67-70 One study showed that a brief educational intervention in the elderly resulted in a significant reduction in alcohol use 24 months later, although health outcomes were not significant.70,71 In general, alcohol detection and intervention is feasible and can reduce heavy alcohol use. More intensive interventions may be necessary for elderly persons with diabetes in order to have an impact on morbidity and mortality.
PSYCHIATRIC MEDICATIONS IN DIABETIC PERIPHERAL NEUROPATHY
Diabetic peripheral neuropathy (DPN) can be defined as “the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after exclusion of other causes.”72 Chronic sensorimotor DPN is the most common presentation of neuropathy in diabetes, and up to 50% of persons with diabetes and sensorimotor DPN experience symptoms of burning, stabbing, electrical or deep aching pain, squeezing, paresthesias, or hyperalgesia. The pain is typically worse at night and classically affects the feet and distal lower extremities, although it may affect the hands as well. Inadequate control of pain commonly contributes to mood and sleep disturbances,73 and treatment of pain has been shown to improve quality of life,74 making this an important part of therapy.
Treatment
Physicians select from a variety of psychotropic agents to treat painful DPN. The only agents currently approved by the FDA for treatment of painful DPN are duloxetine and pregabalin, with pregabalin on the market as a Schedule V drug.
Duloxetine, a reuptake inhibitor of both serotonin and norepinephrine, is thought to inhibit pain via descending pain pathways. In a randomly assigned, placebo-controlled, double-blinded study of 457 patients, 60 and 120 mg per day of duloxetine improved painful DPN by 50% or more compared to placebo, beginning at 1 week and continuing through 12 weeks of treatment.75
The anticonvulsants (gabapentin, pregabalin, topiramate, lamotrigine) appear to be equally efficacious as tricyclic antidepressants, although agents in these drug classes have not been directly compared. Gabapentin and pregabalin have been studied the most. Gabapentin improves pain and sleep disturbances associated with DPN as well as quality of life when taken at doses of 1800-3600 mg/day.74,76 Side effects of gabapentin at these doses include dizziness, somnolence, and confusion. Pregabalin also improves both pain and sleep, in addition to health-related quality of life.77 Downsides include that it requires titration to higher doses, is a 3-times-daily drug, has common side effects of dizziness and somnolence, and is a schedule V drug.
Venlafaxine is another serotonin/norepinephrine reuptake inhibitor that has been tested for painful DPN. Venlafaxine was shown to be effective at doses of 150-225 mg per day, but not at lower doses. The most common side effects were nausea and somnolence, and a significant number of persons exhibited clinically important electrocardiogram changes.78
Tricyclic antidepressants are also thought to relieve pain via inhibition of norepinephrine reuptake at spinal dorsal horn synapses, but also via antagonism of N-methyl-D-aspartate receptors.79 Clinical experience in treating painful DPN is greatest with amytriptyline and imipramine. However, these medications are highly anticholinergic, and desipramine appears to be equally efficacious with fewer side effects.79,80 Effective desipramine doses for treating DPN (10-150 mg per day) are much lower than doses generally prescribed for treating depression. The effect on DPN pain is independent of the antidepressant effect. Clinical use in the elderly is often limited by anticholinergic side effects, orthostatic hypotension, sedation, and risk of cardiac arrhythmias. Approximately one-third of all individuals cannot tolerate these agents.81
Trials of SSRIs for management of painful DPN have been less encouraging. Fluoxetine appears to be no better than placebo.79 Both paroxetine 40 mg per day and citalopram 40 mg per day showed mild efficacy in reducing symptoms of neuropathy as compared to placebo. They appeared to have fewer side effects than imipramine, but less efficacy as well.82,83
CONCLUSION
Older patients with diabetes have a higher risk of comorbid psychiatric disorders that often go undiagnosed. Proper treatment with psychotropic medications in this population is often complex and challenging. With the doubling of the population with diabetes, recognizing and treating dementia, depression, and alcohol abuse in older persons with diabetes will become a greater clinical health care issue. Treating this population will require careful consideration and collaboration among primary care, diabetes, and psychiatry health care providers.
Dr. Feil and Dr. Weinreb have indicated no relevant financial relationships. Dr. Sultzer has received research support from Forest Pharmaceuticals and has been on the speakers bureaus for Pfizer,Inc, Forest Pharmaceuticals, Bristol-Myers Squibb, and AstraZeneca. Research reported in this article was supported by the Department of Veterans Affairs, the VA Greater Los Angeles Health Services Research & Development Center of Excellence, and the Geriatric Research, Education and Clinical Care Center.










