Implications of Pressure Ulcers and Its Relation to Federal Tag 314
INTRODUCTION
The development of pressure ulcers remains a significant health problem in long-term care facilities (LTCFs). The national prevalence rate for pressure ulcers in long-term care is approximately 8.9% according to a federal report from the Centers for Medicare & Medicaid Services (CMS).1 Data from the National Pressure Ulcer Advisory Panel have noted the incidence of pressure ulcers in LTCFs to range from 2.2% to 23.9%.2 To address this health problem, CMS established the reduction of pressure ulcers as one of its goals for the Government Performance and Results Act (GPRA) in nursing homes.3 Moreover, CMS has mandated that each state Quality Improvement Organization address pressure ulcers in long-term care. Thus, it’s evident that CMS believes that the current rates of pressure ulcers in long-term care can be reduced.
Through its regulations, CMS has a significant effect on the standards for pressure ulcer care in long-term care facilities. Any nursing facility that accepts either Medicare or Medicaid reimbursement must comply with CMS regulations. To this end, LTCFs must comply with the federal guidelines for the treatment of pressure ulcers, or they may be subjected to financial penalties or risk having their money withheld, essentially closing the facility. By understanding the revised regulation related to pressure ulcer care, facilities can decrease their exposure to financial penalties.
DEVELOPMENT OF THE REVISED
F-314 CMS selected pressure ulcers as the first regulation to be revised due to its prevalence and high number of citations by surveyors. It should be noted that the federal regulation for pressure ulcers has not changed, only the guidance to state and federal surveyors. The regulation states:
Based on the Comprehensive Assessment of a resident, the facility must: a) ensure that a resident that enters the facility without pressure sores does not develop pressure sores unless the individual’s clinical condition demonstrates that they were unavoidable; b) promote the prevention of pressure ulcer development; c) promote the healing of pressure ulcers that are present (including prevention of infection to the extent possible); and d) prevent development of additional pressure ulcers.4
The revision process of F-314 took approximately 2.5 years to complete. The panel charged with revising F-314 was composed of federal officers, federal and state surveyors, national clinical/academic experts, and key professional groups (National Pressure Ulcer Advisory Panel and American Medical Directors Association). The panel met several times to reinterpret the guidance and held two public hearings. Once the committee’s work was completed, the document was circulated internally at CMS for the appropriate sign-offs. The final revised F-314 Tag document can be read in its entirety at: www.cms.gov.4 The process developed and used for revising F-314 will be used for subsequent revised regulations (urinary incontinence [F-315], accidents [F-325], etc)
HIGHLIGHTS OF PRESSURE ULCER TREATMENT
The revised F-314 comprises 40 pages, including references and the investigative protocol for surveyors. F-314 is divided into three sections: (1) definitions, (2) prevention, and (3) treatment. More specifically, the treatment section covers such topics as assessment of pressure ulcers, staging, infection, pain, and dressings. When the surveyor completes the survey process for residents with pressure ulcers, the surveyor should be able to determine the effectiveness of pressure ulcer care. Moreover, they should be able to determine whether the care provided lead to an avoidable or unavoidable progression of the ulcers.
Pressure ulcer avoidability is defined by CMS as follows: “The resident developed a pressure ulcer, and the facility did not do one or more of the following: evaluate the resident’s clinical condition and pressure ulcer risk factors; define and implement interventions that are consistent with resident needs, resident goals, and recognized standards of practice; monitor and evaluate the impact of the interventions; or revise the interventions as appropriate.” Unavoidability is defined as: “The resident developed a pressure ulcer even though the facility had evaluated the resident’s clinical condition and pressure ulcer risk factors; defined and implemented interventions that are consistent with resident needs, goals, and recognized standards of practice; monitored and evaluated the impact of interventions; and revised the approaches as appropriate.” Hence, CMS has clearly placed the onus of providing quality pressure ulcer treatment with the LTCF.
Treatment
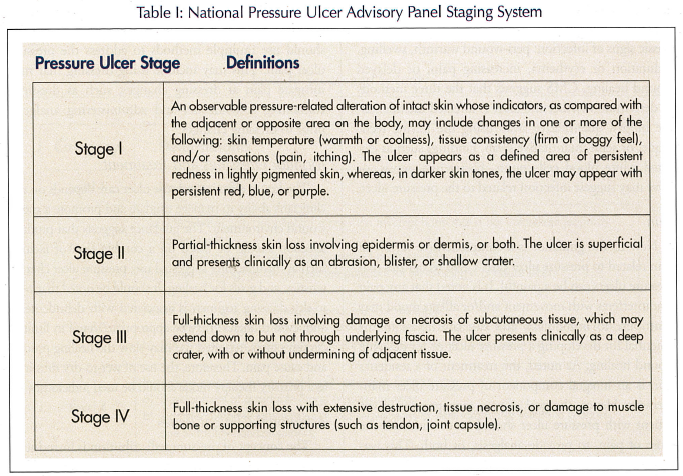
The treatment of pressure ulcers is predicated on the fact that clinical staff can properly assess ulcer characteristics. Hence, the clinical staff can: (1) differentiate the type of ulcer (pressure versus non-pressure-related ulcer, since interventions can vary depending on ulcer type); (2) determine the ulcer’s stage (using the National Pressure Ulcer Advisory Panel [(NPUAP)] Staging System); (3) describe and monitor the ulcer’s characteristics; (4) monitor the progress towards healing and for potential complications; (5) determine if infection is present; (6) assess, treat and monitor pain, if present; and (7) monitor dressings and treatments.
Differentiate the Type of Ulcer (pressure versus non-pressure-related ulcer)
The Guidance is clear that the LTCF should have a system in place to assure that the protocols for daily monitoring, periodic documentation of ulcer measurements, terminology, frequency of assessment, and documentation are consistently implemented throughout the facility. Hence, the facility should have a systematic plan in place to assure standardized pressure ulcer care.
The LTCF should have staff members who are proficient in their ability to delineate between a pressure ulcer and the more common chronic ulcers: venous insufficiency ulcers, arterial insufficiency ulcers, and neuropathic ulcers. Moreover, the guidance suggests that the clinical staff should document the clinical basis for any determination that an ulcer is not pressure-related, especially if the ulcer has characteristics consistent with a pressure ulcer. Hence, the staff should have a clear and consistent documentation to confirm other types of chronic ulcers.
Determine Ulcer’s Stage and Ulcer Characteristics
A good discussion is noted regarding the assessment of the ulcer, either at each dressing change or at least weekly. The minimum documentation should include the location of the ulcer and its stage, using the NPUAP Staging System (Table I). The size of the ulcer should be noted (length, width and depth). Pain should be evaluated if present. Moreover, the clinician or wound care nurse should assess both the nature and frequency of the pain. Further, documentation should note the presence of exudate, characteristics of the wound bed, and the description of the wound bed. CMS supports the use of photographs if the facility has developed a protocol consistent with accepted standards.
Although CMS supports the use of photographs, there are pros and cons of using this technology that should be considered. The NPUAP suggests that some of the advantages of photography include: a visual confirmation to the written record; demonstration of a wound imported to the facility mitigates liability concerns; and protocols for photography further ensure that all body surfaces at risk of skin breakdown are viewed during the admission exam.5 Conversely, some of the disadvantages include: if camera is used only on admission, it invites inconsistent application and undermines adherence to standard procedures; a photograph taken at a single point in time may undermine faith in the treatment plan if the ulcer worsens; consistency of staff taking photographs underscores the need for staff training; and photographs of large pressure ulcers can be inflammatory in a jury trial, despite compliance with all reasonable standards.5
Monitor the Progress Towards Healing and for Potential Complications
The guidance to surveyors supports the use of the Resident Assessment Instrument (RAI), which includes directions to describe the healing ulcer. Although CMS understands that pressure ulcers do not heal from a Stage IV to a Stage III (eg, reverse staging) until the Minimum Data Set (MDS) is changed, reverse staging ulcer should continue. New to the guidance is a discussion on the time frame for staff to reassess the pressure ulcer for healing. It is noted that if the clinician does not observe progress towards healing within 2-4 weeks, the staff needs to reassess the resident and ulcer.
Because of this new guidance, the role of the nursing staff to consistently assess and document pressure ulcers is critical to accurately delivering appropriate wound care. The nursing staff must be vigilant in monitoring the wounds on a daily basis (ensure that dressing and treatments are being implemented as ordered) and doing weekly measurements to determine the healing or deterioration of the ulcer so that more appropriate treatment can be considered.
Determine If Infection Is Present
Infection has become a major concern for CMS. The guidance recognizes that all Stage II – IV pressure ulcers contain colonies of bacteria, but may not be infected. Clinicians should pay particular attention to classic signs of infection: peri-wound warmth, swelling, induration or erythema, increasing pain, or delayed wound healing. CMS suggests that the three methods to determine whether the ulcer is infected include: tissue biopsy, quantitative swab using the Levine technique, or semi-quantitative swab. Findings such as elevated white blood cell counts, bacteremia, sepsis, or fever may suggest infection related to the pressure ulcer.
Pain
New to the guidance is a comprehensive review of pain related to pressure ulcer care. CMS recognizes that pressure ulcers can be a painful. It is noted that any pain that interferes with movement and/or affects mood may contribute to immobility, and can contribute to the potential for developing a pressure ulcer or for delaying wound healing. As noted, the treatment of a resident’s pain is an integral component of pressure ulcer management. Hence, the goal of pain management in the patient with pressure ulcer should be to eliminate the cause of pain, to provide analgesia, or both. This was supported recently by the World Union of Wound Healing Societies consensus document, Principles of Best Practice: Minimizing Pain at Wound Dressing-related Procedures.6 The document emphasizes that unresolved pain negatively affects wound healing and has an impact on quality of life. Pain at dressing-related procedures can be managed by a combination of accurate assessment, suitable dressing choices, skilled wound management, and individualized analgesic regimens. When it comes to dressing changes, the World Union consensus document makes the claim that it is important for the clinician to recognize the potential to cause pain at dressing changes. Dressing removal can potentially cause damage to delicate tissue in the wound and surrounding skin. This document further states that dressing selection is important, and that the following parameters should be considered: maintain moist wound healing; use a dressing that is atraumatic to the wound and surrounding skin; absorbent capacity; and allergy potential.
CMS recognizes the subjective nature of pain, so objective tools to elicit resident’s pain should be used. CMS also notes that residents may experience pain of varying intensity and frequency. Thus, clinicians should use multiple methods to address the pressure ulcer pain. This may include using dressing that may mitigate pain at dressing changes, such as dressings containing soft-silicone, and administering analgesic prior to dressing changes.
Monitor Dressings and Treatment
The key to effective pressure ulcer care depends on the clinician’s ability to manage exudate and promote a moist wound environment. The guidance suggests that product selection should be based on a combination of factors, such as manufacturer suggested use, pressure ulcer characteristics, and goals for healing. It should be noted that wet-to-dry dressing regimen is associated with debridement, and, even though it may be appropriate to use in limited circumstances, repeated use may slow the healing process and cause pain. Therefore, the use of wet-to-dry dressings as a primary therapy mode should be used judiciously.
Additional Clinical Issues
The concept of pressure redistribution is introduced for the first time in the guidance. When pressure is reduced in one anatomical location, it may increase the pressure gradient in another anatomical location. Therefore, clinicians and nursing staff need to be cognizant of relieving/reducing the pressure. Further, parameters for turning and repositioning are provided. Residents in beds should be turned and repositioned minimally every two hours, and minimally every hour when sitting in a chair, independent of support surface.
Finally, the guidance supports the resident’s right to refuse one or more aspects of pressure ulcer care. However, the guidance is clear that an active advance directive does not translate into providing less pressure ulcer care. The clinician must provide pressure ulcer care that is congruent with resident and/or family wishes.
INVESTIGATIVE PROTOCOL AND DEFICIENCY CATEGORIZATION
Investigative Protocol
The investigative protocol is used by federal and state surveyors in two ways: (1) to determine pressure ulcer avoidability or unavoidability; and (2) to determine the effectiveness of the long-term care facility in preventing and treating pressure ulcers. The surveyors determine compliance with acceptable treatment standards of practice through direct observation, resident/staff interviews, medical record review, care plan review, and interviews with health care practitioners and professionals. If the survey process determines deficient practice by the LTCF, then the surveyor along with the state agency determines the level of deficiency.
Deficiency Categorization
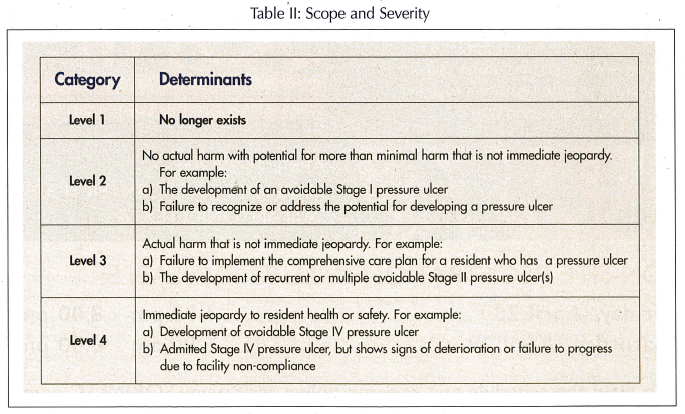
To determine the level of deficiency, the survey team must consider three important elements: (1) presence of harm/negative outcome(s) or potential for negative outcomes because of lack of appropriate treatment and care; (2) degree of harm (actual or potential) related to the noncompliance and; (3) the immediacy of correction required.
Upon evaluating the three elements, the survey team determines the level of severity. The revised F-314 has eliminated Level 1 deficiencies, since the agency believes that the development of a pressure ulcer is more than minimal harm. Thus, any noncompliant LTCF must be cited minimally at Level 2 (no actual harm, with potential for more than minimal harm that is not immediate jeopardy). Further, the document now includes examples of deficiencies for each level of scope and severity (Table II). This was developed to decrease the variability between surveyor deficiencies within and between states for similar noncompliance infractions.
CONCLUSION
The treatment of patients with pressure ulcers remains challenging. The revised F-314 provides clearer guidance not only to the surveyors, but to long-term care facilities. Although the bar has been raised on CMS’s expectation of resident care related to pressure ulcers, they provide helpful information to achieve the expected outcomes. This new evidence-based approach to the guidance should ensure improved quality of care for our residents, which is everyone’s goal.
Research reported in this article was funded by an unrestricted educational grant from 3S.
The author reports no relevant financial relationships.










