Anticoagulation in a High-Risk Nursing Home Resident
INTRODUCTION
Atrial fibrillation is a common cardiac arrythmia in the geriatric population.1-4 It is of significant concern because of its association with increased risk of stroke. Patients with atrial fibrillation are six times more likely to suffer a stroke, and this outcome is more common with advancing age, which is, in itself, an independent risk factor.3,5,6 Multiple studies published since the 1990s and clinical practice guidelines strongly endorse the use of anticoagulation for nonrheumatic atrial fibrillation in patients at high risk for stroke.5-8 Although warfarin is beneficial, the increased risks of intracranial hemorrhage and gastrointestinal and genitourinary bleeding make its usage a difficult clinical decision.2,3,5,9 Once the decision to anticoagulate has been made, the physician is obligated to maintain anticoagulation in a narrow range. The physician must be aware of any new drug interactions (ie, newly started antibiotics, antidepressants, or nonsteroidals), patient preferences (ie, diet, smoking, alcohol), medication compliance, and any other factors that may lead to difficulty in maintaining a narrow therapeutic window.
The following is a case of a nursing home resident with multiple comorbidities, recent illness, and multiple physicians, the combination of which resulted in the resident suffering a stroke and eventual death, while subtherapeutic during anticoagulation. This case especially illustrates multiple factors that may have led to poor patient safety with the use of warfarin.
CASE DESCRIPTION
An 83-year-old male resident of a nursing home was found unresponsive in his room at 12:15 pm. His vital signs were: blood pressure, 120/99; pulse, 95; blood glucose, 56; respiration rate, 16; and oxygen saturation rate, 98%. On nursing assessment, physical examination was remarkable for right facial drooping. The resident was transferred urgently to the hospital where he died later that week from complications of a stroke.
This resident lived in the nursing home for the last three years with several intermittent hospitalizations for congestive heart failure, pneumonia, and urinary tract infections. He was wheelchair-bound secondary to multiple sclerosis, was bed-bound during episodes of illness, and had a chronic indwelling Foley catheter for neurogenic bladder. He was very thin and appeared malnourished. He had a history of weight loss secondary to poor eating because of a dislike of the nursing home diet. (On nutritional evaluation, he was recommended for a puréed diet due to some difficulty with swallowing. His family brought him food from home to compensate.) The resident was known to have a cardiac examination of regular rate, a sinus rhythm with a 2/6 systolic ejection murmur nonradiating, a usual heart rate of 70-80, and blood pressure ranging from 100/60 to 128/70. He had no bruits, rubs, or gallops, and no S3 or S4. His lungs were recently clear with no rales or rhonchi. He was also noted to have two pressure ulcers, one on the sacrum and one on the heel.
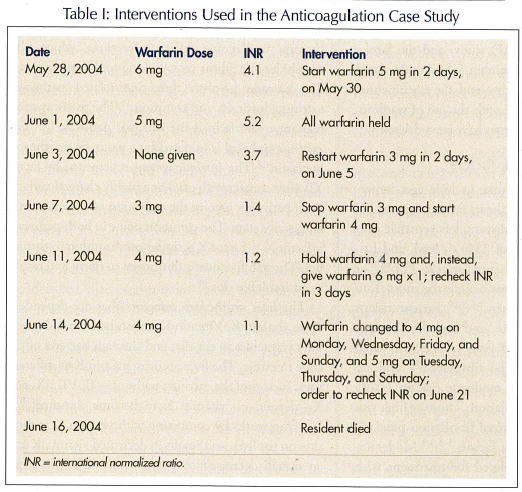
In the two weeks preceding this unresponsive episode, the patient was noted to have a urinary tract infection and was treated with a 10-day course of trimethoprim/sulfamethoxazole twice a day. He was also given hydroxyzine 10 mg once a day for 5 days for a pruritic rash. The antibiotic was stopped on May 30, 2004. The hydroxyzine was stopped on May 27, 2004. Prior to his urinary tract infection, international normalized ratios (INRs) measured from October 21, 2003 to May 28, 2004 were predominantly subtherapeutic, with three supratherapeutic values and only one therapeutic value. The INR values starting from October 21 to May 28 were 1.13, 1.81, 1.20, 1.32, 1.22, 1.45, 1.53, 1.71,1.95, 4.9, 5.1, 1.2, 1.5, 2.4, and 4.1. The resident’s anticoagulation status was evaluated on May 28, 2004, due to his illness and the possibility of drug interactions with trimethoprim/sulfamethoxazole. This was remarkable for the variable results that were noted over the next two weeks and the many changes in medication by different physicians in an attempt to gain control. Table I illustrates the dates, dosing schedule, INRs, and interventions during the course of this case.
The resident had a past medical history of chronic nonvalvular atrial fibrillation, multiple sclerosis, neurogenic bladder with frequent urinary tract infections, chronic obstructive pulmonary disease, repair of an abdominal aortic aneurysm in 1997, cholelithiasis and resultant cholecystectomy, two pressure ulcers (one on the sacrum and one on the heel), and renal insufficiency. His medication regimen consisted of a daily multiple vitamin, a stool softener, zinc 220 mg, vitamin C 500 mg twice a day, gabapentin 400 mg (two capsules) three times a day for neuropathy, albuterol via nebulizer, diazepam 2 mg at bedtime for muscle spasms, oxygen as needed for shortness of breath, and acetaminophen 650 mg as needed. His social history was significant with a lifelong history of cigar smoking, which he continued to do in the nursing home.
DISCUSSION
When utilizing anticoagulants in the elderly for nonval-vular atrial fibrillation, the physician must consider several factors with regard to patient safety. The first factor is whether an individual qualifies for anticoagulation, based on current guidelines.3-5,7,9 The evidence for these guidelines is derived from numerous studies. Based on a consensus of six primary prevention trials—the Atrial Fibrillation, Aspirin, Anticoagulation Study (AFASAK) from Copenhagen; the Stroke Prevention in Atrial Fibrillation (SPAF-I) study; the Boston Area Anticoagulation Trial in Atrial Fibrillation (BAATAF); the Canadian Atrial Fibrillation Anticoagulation (CAFA) study; the Veterans Affairs Stroke Prevention In Nonrheumatic Atrial Fibrillation (SPINAF) study; and the Stroke Prevention Using ORal Thrombin Inhibitor In Atrial Fibrillation (SPORTIF) study—and the documented 68% risk reduction of stroke with the use of warfarin, several risk stratification schemes have been devised.3,4,6
Stratifying Individual Risk Factors
Major risk factors for stroke include age, hypertension, diabetes, congestive heart failure, myocardial infarction, coronary artery disease, left ventricle dysfunction (ejection fraction of 35% or less), and history of prior transient ischemic attacks (TIAs) or stroke.5,8,9 High-risk residents benefit the most from anticoagulation with warfarin.1-3,9,10 Patient risk is categorized as low, moderate, and high.8,9 Those at low risk are those under 65 years of age with atrial fibrillation only (“lone atrial fibrillation”) and no other risk factors. Those at moderate risk are 65-75 years of age with atrial fibrillation. Those at high risk are 75 years or older with atrial fibrillation plus any of the above-mentioned risk factors.1-3,5,9 Moderate-risk residents can be considered for treatment with either warfarin or aspirin, although warfarin is now preferred. Low-risk patients can be considered for aspirin alone.5,9 The combination of aspirin and clopidogrel has been used in individuals who are unable to take warfarin, but this has not been a widely researched topic. An easy mnemonic for stroke risk factors is CHADS.5,6 These risk factors include Congestive Heart failure, Advanced age (over age 75), Diabetes, and previous Stroke. One point is assigned to each of these factors and two points for a previous stroke.
Committing to Warfarin: Understanding the Coagulation Cascade
Once the decision has been made to anticoagulate with warfarin, one must take various factors into consideration. (Consider the influences on maintenance of a therapeutic INR.) Understanding the coagulation cascade helps the physician to understand warfarin’s mechanism of action, its role in anticoagulation, its influences on the maintenance of an INR, and the method and parameters for monitoring. The coagulation cascade is made up of a series of blood-clotting proteins undergoing different reactions, which ultimately lead to fibrin cross-linking and coagulation.11 The cascade involves three interrelated pathways: extrinsic, intrinsic, and common.11 The pathway most relevant to warfarin is the extrinsic pathway.10,12 The extrinsic pathway is initiated by a tissue factor, thromboplastin.11 The intrinsic system is activated by factor XII after contact with glass or a highly charged surface. Both pathways involve the activation of enzymes from an inactive state. The common point in both pathways is factor X.11 Factor X activates prothrombin to thrombin. Thrombin converts fibrinogen to fibrin and results in an insoluble clot.11
The liver synthesizes cofactors that are dependent upon vitamin K. Vitamin K is absorbed through green leafy vegetables in the diet and through bacteria of the large intestine. The liver absorbs vitamin K at different sites. Factors of the extrinsic pathway—II, VII, IX, and X—depend on vitamin K to become activated.10-12 Warfarin works by competing with vitamin K at these sites on the liver, and results in decreased vitamin K and an overall decreased coagulation.11,12 Warfarin treatment specifically results in inactivating clotting factors because of a reaction in which glutamic acid residues are carboxylated and form alpha carboxyglutamic acid residues.10-12 Carboxylase, which depends on vitamin K, forms a new COOH group on glutamic acid, and the reduced vitamin K cofactor is converted to vitamin K epoxide (by vitamin K epoxide reductase).10 Vitamin K epoxide reductase is the enzyme that is inhibited by warfarin.10 The clotting factors are now inactivated since they lack the alpha carboxylglutamyl sidechains.10 Clinically, this means that when an INR is supratherapeutic, vitamin K can be used to overcome the warfarin at these sites, and thus lower the INR.
Monitoring Warfarin
Because warfarin does not affect previously circulating coagulation factors, depletion of these factors must occur before warfarin acts. Each factor of the extrinsic path has a different half-life. For example, factor II requires 60 hours, factor VII requires 4-6 hours, factor IX requires 24 hours, etc.12 Because of the delay with these half-lives, several days of therapy are needed in order to have a complete clinical response.12 Warfarin effects are not observed for 8-12 hours, and reversal can take up to 24 hours.10,12 Warfarin is rapidly and completely absorbed orally, and it is 99% bound to plasma albumin.10 This binding to albumin is the basis for many of warfarin’s drug interactions.10,12 At each step of the cascade, feedback mechanisms regulate the balance between active and inactive enzymes.12 In the past, warfarin was monitored by the prothrombin time (PT). The test involves centrifuging the patient’s blood into plasma and clotting proteins, and thromboplastin is added. Clotting would take place in 11-13 seconds; however, warfarin will cause clotting to take longer.13 It was detected that prothrombin values were different from one laboratory to another, and so a standardization method was developed.13 This standardization method is known as the INR and allows PTs to be compared from one laboratory to another.13
Another anticoagulant that is sometimes used is heparin. Heparin is used when a rapid anticoagulant effect is needed. Heparin works via the intrinsic pathway. Heparin inactivates clotting factors II, IX, X, XI, XII, and XIII.10 Heparin acts indirectly by binding to antithrombin III.10 When heparin binds to antithrombin III, a molecular change occurs, which allows thrombin to be rapidly inhibited and prevents fibrin formation. Without heparin, antithrombin III interacts slowly with thrombin.10 Heparin has the advantage of quick onset and quick reversal of effects when the drug is stopped. Similar to warfarin, heparin is associated with bleeding complications. Heparin is monitored through the activated partial thromboplastin time (PTT) so that it is 1.5-2.5 times the normal control.10 Protamine sulfate inactivates heparin.10
Although both warfarin and heparin are associated with bleeding, and both require monitoring of hemoglobin and hematocrit for signs of blood loss, heparin may also affect platelets.10 A decrease in platelets may occur after eight days. Some patients may develop antiplatelet antibodies and possibly heparin-induced thromboembolism.10 In this case, a new anticoagulant would have to be started. Renal insufficiency and hepatic cirrhosis can increase the half-life of heparin, and therefore increase bleeding risk.10
Medication Interactions
Several medications that inhibit vitamin K and increase sensitivity to warfarin include amiodarone, cimetidine, disulfiram, metronidazole, sulfa-derivatives, second- and third-generation cephalosporins, nonsteroidal anti-inflammatory drugs, vitamin E, clofibrate, statin compounds, and heparin.10 Sulfonamides have a greater affinity for the binding site and can transiently displace this anticoagulant.10,12 Other medications increase vitamin K absorption and cause a decreased sensitivity to warfarin,10,12 including barbiturates, carbamazepine, ethanol, cholestyramine, and rifampin. Medical conditions that increase the anticoagulant effect of warfarin include hyperthyroidism, liver disease, low dietary vitamin K, malnutrition (decreased albumin and reduced protein binding), reduced vitamin K absorption, renal insufficiency, and hepatic insufficiency. Diets rich in green leafy vegetables and some nutritional supplements cause increased vitamin K absorption and a subsequent decrease in warfarin sensitivity. Some herbal supplements, including garlic and ginkgo, also increase sensitivity to warfarin.10,12
Patient Factors
One must also consider risk factors in the elderly, such as falls, dementia, and altered pharmacokinetics and pharmacodynamics, in order to balance the risk of intracranial hemorrhage (due either to a spontaneous intracranial hemorrhage or to a head injury from a fall), with the benefit of prevention of ischemic stroke. Much of the literature cites that the benefit of stroke prevention far outweighs the risk of intracranial bleed.5,7,9 However, certain factors must be considered on an individual resident basis. Intracranial hemorrhage occurs more often in neurologic conditions and severely uncontrolled hypertension. Although falls can result in intracranial hemorrhage, most falls result in minor injuries, so this is not as strong a contraindication as once thought. Dementia results in difficulty in following directions and compliance (more of an issue for the patient living independently than for a resident of a nursing home).
Pharmacokinetic issues that commonly affect the elderly—chiefly renal insufficiency and decreased hepatic metabolism—play an important role in increasing sensitivity to warfarin.10,12,14 Other contraindications include bleeding disorders, liver disease, uncontrolled hypertension, vitamin K deficiency, frequent bleeding episodes from the gastrointestinal or genitourinary systems, and cerebral vasculopathy. Quality of life is also a consideration, and therefore warfarin is not recommended in those with terminal illness or cancer, in whom the prognosis is poor.
Calculating the Right Dose
Monitoring the INR in atrial fibrillation is critical. It must be maintained in the moderate-intensity range of 2.0-3.0. Intracranial hemorrhage is often associated with high-intensity anticoagulation, which occurs when the INR is maintained at or ventures above 4.0 or higher.2,7,12,14
Adjustments in warfarin dosing should be made on the basis of the week’s dosage. Warfarin has a long half-life ranging from 36-72 hours, adding to the difficulty in monitoring and adjusting dosages rapidly. When adjustments in the dosage are indicated based on the INR, it is recommended that the weekly dose be increased/decreased 5-15%.10,12 Initial increases of 10-20% can be made on day 1, then 5-10% changes are made every 4-7 days. Once the resident is stable, the physician should monitor the INR weekly, and then monthly once it is at a therapeutic level. If the resident’s INR is supratherapeutic, from 3.1-3.9, initial decreases of 5-10% can be made. If the INR is > 4, the current warfarin dosage should be maintained at least 1 day before starting a lower dosage. If the INR is > 5, the administration of intramuscular or intravenous vitamin K must be considered, and the patient should be monitored closely for bleeding.10,12,14 Drug interactions, dietary indiscretions and contraindications (green leafy vegetables), patient compliance issues (ie, refusing doses), and physician and staff communication must all be considered in monitoring.
Specialized Anticoagulation Services
As this case clearly illustrates, residents on warfarin require close monitoring and careful attention on the part of the physician. New literature and studies examining the risk and benefit of anticoagulation in light of safety and medical error concerns, as well as outcome-based practices, have proposed specialized anticoagulation services as a means to maximize benefit and limit risk.15-17 These services begin with physician referral, describing the type of anticoagulation desired and the appropriate therapeutic range desired.15,17 The services also include a dedicated anticoagulation clinic, a team of nonphysicians including a nurse, a pharmacist, and other health workers; specialized computer software; frequent communication with the resident; and assistance with home monitoring for patients living independently.15-17 With these services, the physician is always kept informed of the INR; however, adjustments are made by the team or clinic.15-17 In some medical settings, patients may exhibit more control over their condition by using fingertip monitoring at home and reporting to a telephone service, as opposed to reporting to a laboratory and a phlebotomist.6,15,16
NEW AND FUTURE TRENDS: PROPAGATION INHIBITORS, DIRECT THROMBIN INHIBITORS, AND USE OF PERIOPERATIVE ENOXAPARIN
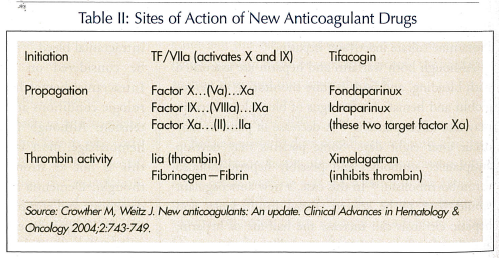
Ximelagatran was presented to the Food and Drug Administration (FDA) in 2003 as an anticoagulant with similar efficacy and bleeding risk to warfarin, and as a fixed-dose medication with no necessity for monitoring. However, it was found to cause liver toxicity and some subsequent deaths, and has not yet been approved.4 Fondaparinux, a once-daily injection, is currently available, and idraparinux, a once-weekly injection, is still in trials.18 Oral factor Xa inhibitors are still in early trials. Table II illustrates the sites of action of the new anticoagulant medications. If warfarin is stopped in a high-risk patient before surgery, guidelines (not the FDA) suggest that low-molecular-weight heparin can be used up to 12 hours before a procedure; once the patient becomes stable after the procedure it can be started until the INR is therapeutic once again.19 It is important to note that enoxaparin is not FDA-approved for perioperative use and is used anecdotally. There is a strong warning against perioperative use of enoxaparin in patients with valvular atrial fibrillation.19
Surgical interventions, catheter interventions, pacemakers, defibrillators, and echocardiography are also new and exciting options that can be explored in appropriate individuals.
CASE CONCLUSIONS
In the case described in this article, several complicating factors may have contributed to the initial supratherapeutic INR, the subsequent subtherapeutic INR, and the resident’s eventual death. The resident’s record showed that his INR—which should be monitored and adjusted at least monthly—had been drawn but not followed. Of all the values noted in the chart, only one value was therapeutic. Monitoring resumed only when the resident was treated for a urinary tract infection. The antimicrobial medication that was administered, trimethoprim/sulfamethoxazole, is known to increase the effect of warfarin and prolong the INR. The patient was also known to have renal insufficiency, which plays a role in increasing the INR through decreased clearance of the drug and prolonged serum half-life.
Factors that contributed to the difficulty in increasing the subtherapeutic INR included the resident’s noncompliance with diet, the long half-life of the drug (32-72 hours for warfarin), laboratory adjustments, and medication adjustments made only every 3-4 days and appearing random instead of following an algorithm or set pattern. There was also inconsistent communication among physicians. In addition, the resident continued smoking cigars in the nursing home, thus compounding his risk of stroke.
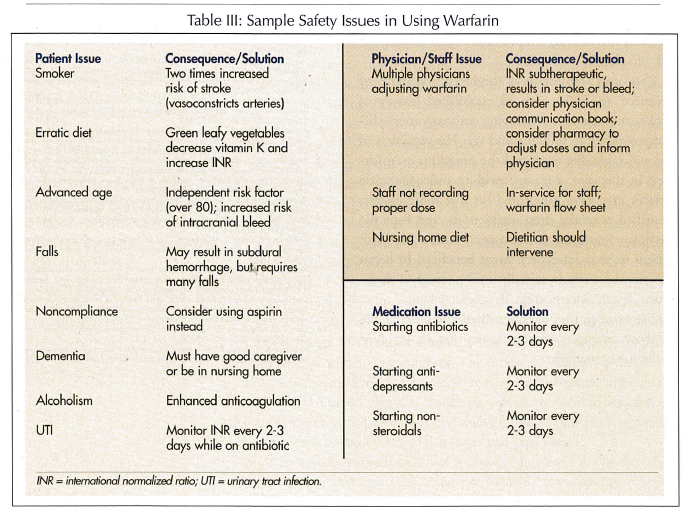
This case represents a high-risk resident of advanced age (80s) with an established history of stroke, and probable underlying coronary artery disease secondary to smoking and age. His variable and unpredictable diet depressed the prognosis even further. In this case, a team providing anticoagulation services may have monitored his diet more closely, identified potential drug interactions, and kept the physician informed. In retrospect, with a high-risk patient, it may have been more beneficial to begin heparin and warfarin initially until he reached therapeutic levels. Monitoring, if conducted in a more precise manner, may have benefited outcomes. Table III shows examples of safety issues that are involved in the use of warfarin.
CONCLUSION
Anticoagulation in the appropriately selected individuals with atrial fibrillation has been shown to prevent 68% of ischemic strokes.1-3,5,9 Physicians often underutilize anticoagulation in atrial fibrillation because of the difficulties of monitoring patients, maintaining them at a therapeutic range, and the perceived increase in risk in frail, elderly patients.14,15,17 Although anticoagulation is never without risk, new advances, such as warfarin clinics, anticoagulation teams, home monitoring, and newer agents, may make anticoagulation safer in the future.9,15-17










