Anemia, Fatigue, and Aging
Aging is associated with a progressive decline in the functional reserve of multiple organ systems, and in personal and social resources.1 This combination leads to an impaired stress-coping ability. Although some changes of aging are unavoidable, many more may be delayed or prevented. Death is not preventable, but ideally, morbidity and disability may be delayed. “Compression of morbidity” is the main goal of geriatrics.2
Correction and prevention of anemia may play a role in compression of morbidity.3 Anemia is a cause of hypoxia, which limits the physical activity and impairs the synthesis of new proteins, leading to sarcopenia and increased disease susceptibility, which in turn, may lead to more anemia. This vicious cycle appears to be maintained by catabolic cytokines, which are a hallmark of aging and are associated with most geriatric syndromes, from dementia to osteoporosis to failure to thrive.4-8 In addition, anemia may be the first sign of lethal, but potentially curable, diseases including B12 deficiency or cancer of the digestive or urogenital tract. This article explores the implications of anemia in older patients and, in particular, in older cancer patients.
EPIDEMIOLOGY AND CAUSES OF ANEMIA IN OLDER AGE
The incidence and prevalence of anemia increase with age. The Third National Health And Nutrition Examination Survey (NHANES III) found a prevalence of anemia higher than 10% in individuals age 65 years and older.9 That prevalence increased with age, and was higher for African Americans, when compared with other ethnic backgrounds. Anemia was more common in older men than in older women, but this finding should be qualified. According to the World Health Organization (WHO), the normal hemoglobin values are 12 gm/dL or higher for women, and 13.0 gm/dL or higher for men. These values have been criticized since the publications of the Women’s Health and Aging Studies (WHAS), which demonstrated that in women age 65 years and older, hemoglobin levels lower than 13.5 gm/dL were associated with increased risk of mortality10 and functional impairment.11 If the average hemoglobin levels are the same in older men and postmenopausal women, the prevalence of anemia in NHANES III was similar for both sexes.
The Olmstead County studies demonstrated an age-related increase in incidence and prevalence of anemia.12 The prevalence of anemia was somewhat higher in Olmstead County, as this was a survey of the full population, including the sickest and oldest individuals. An Italian cross-sectional study showed a prevalence of anemia of 9.2% for individuals age 65 years and over, which increased with age.13 While the prevalence of anemia increased with age, the average concentration of hemoglobin remained stable in individuals age 85 years and over in all three studies, indicating that anemia is not an expected consequence of age.
The most common causes of anemia in older individuals in NHANES III and the Olmstead County study are shown in Table I. It is possible that more investigations might have revealed more specific causes for the so-called “anemias of unknown causes,” including early myelodysplasia and anemia of renal insufficiency.
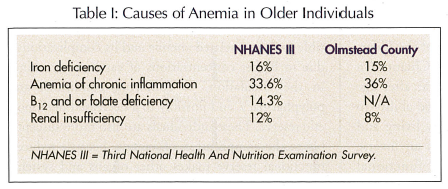
Recent findings are germane to the discussion of the causes of anemia in older individuals:
• Incidence and prevalence of B12 deficiency increase with age.14,15 The most common cause of B12 deficiency is the inability to digest food containing B12 due to decreased gastric secretion of hydrochloric acid and of pepsin; this may be responsive to oral crystalline B12. In addition to anemia, B12 deficiency may be a cause of neurologic disorders, including dementia and posterior column lesions.16
• Seemingly, the main cause of iron deficiency is chronic bleeding from cancer, diverticuli, or angiodysplasia. In older age, iron deficiency may have other causes, including decreased absorption of iron due to gastric achylia, and to increased circulating concentrations of hepcidin. Hepcidin prevents the absorption of iron from the duodenum, and is a protein that is synthesized in the liver, and its production is stimulated by interleukin-6 (IL-6).11,17
• In some older individuals, the secretion of erythropoietin, and the erythropoietic response to erythropoietin, may be impaired as a result of increased circulating concentrations of IL-6 and other inflammatory cytokines.18 Conditions of absolute or relative erythropoietin deficiency, the first due to reduced glomerular filtration rate, the second to increased concentration of proinflammatory cytokines, seem to have a prominent role in anemia of aging.
CONSEQUENCES OF ANEMIA
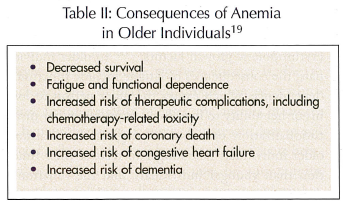 The clinical consequences of anemia are listed in Table II.19 At least four cohort studies demonstrated that anemia is an independent risk factor for mortality in older individuals.10,20-22 Of these, the most provocative are WHAS, which reported an increased risk of mortality for hemoglobin levels lower than 13.4 gm/dL, and may mandate a revision of the WHO definition of anemia in older women.10
The clinical consequences of anemia are listed in Table II.19 At least four cohort studies demonstrated that anemia is an independent risk factor for mortality in older individuals.10,20-22 Of these, the most provocative are WHAS, which reported an increased risk of mortality for hemoglobin levels lower than 13.4 gm/dL, and may mandate a revision of the WHO definition of anemia in older women.10
A serious consequence of anemia is functional dependence.11,23,24 The WHAS, the Established Populations for Epidemiologic Studies of the Elderly (EPESE), and the Invecchiare in Chianti (In CHIANTI) study all demonstrated that after age 65, anemia was associated with decreased ability to perform instrumental activities of daily living and with mobility impairments. There was an inverse linear relationship between risk of functional dependence and mobility impairment, and hemoglobin levels lower than 13.5 gm/dL, indicating that even mild anemia may have serious health and social consequences.
Anemia is associated with increased risk of therapeutic complications. At least four studies showed that anemia was an independent risk factor for the complications of drug therapy,25-28 due to increased concentrations of free drug in the circulation,25 and to chronic hypoxia of normal tissues.29
The association of chronic anemia and congestive heart failure is well known. A recent review of Medicare records showed that individuals age 65 years and older with myocardial infarction and hematocrit levels lower than 30% were more likely to die if they did not receive any blood transfusions.30
A titillating possibility is that anemia may cause cognitive impairment. A study of patients with chronic renal failure showed that cognitive decline was more common among patients whose anemia had not been corrected with erythropoietin.31 Jacobsen et al32 showed a correlation between hemoglobin levels and performance of three cognitive tests in women with cancer who were receiving chemotherapy.
ANEMIA AND CANCER
Anemia is present in 15-50% of patients with cancer at diagnosis. Anemia is more common in neoplasms that involve the bone marrow (eg, leukemia, myeloma), and in those associated with chronic bleeding, such as cancer of the gastrointestinal tract.33,34 The incidence of anemia increases with the progression of cancer, and the causes include myelosuppression from cancer chemotherapy, sarcopenia, bleeding, and increased concentration of catabolic cytokines in the circulation. Older individuals are particularly susceptible to cancer-related anemia and its complications,3 due to higher concentrations of catabolic cytokines in the circulation, to comorbid conditions and polypharmacy, and possibly to a decreased hemopoietic reserve. These individuals are also more susceptible to the complication of anemia, because reduced functional reserve makes other organs and systems more vulnerable to anoxia.
The consequences of anemia in the older cancer patient include increased risk of chemotherapy-related complications and fatigue.
As the majority of cytotoxic agents are bound to red blood cells, a reduction in hemoglobin is associated with increased concentration of free drug in the circulation, and with increased risk of therapeutic complications.25 In younger patients, the effects of anemia may be buffered to some extent by other tissues. In older patients with sarcopenia, this compensatory mechanism is less efficient. Compounds whose toxicity may be increased in the presence of anemia include anthracyclines, epipodophyllotoxins, alkaloids, and camptothecins.28 Fatigue is a sense of physical exhaustion that does not recover with rest, and that impairs the ability to conduct daily activities.35
Fatigue is the most common chronic symptom of cancer patients receiving cytotoxic chemotherapy, and is a cause of social dysfunction. In a recent study, 40% of cancer patients suffered from fatigue, and almost 10% of their caregivers had to quit their jobs, with many more having to curtail their working hours due to fatigue.36 Anemia is the main cause of fatigue in cancer patients, as demonstrated by numerous studies in which fatigue, social activities, and overall quality of life were improved when anemia was corrected with erythropoietic growth factors.37-39 In older individuals, the consequences of fatigue include functional dependence, which causes the older person to lose his or her ability to live alone, requiring either institutionalization or a home caregiver. In addition, older individuals take longer to recover their function than younger individuals, and the functional dependence may become permanent.3
Fatigue is an indication to reverse anemia in cancer patients, when hemoglobin levels are lower than 12 gm/dL. Other forms of management of fatigue, in addition to correction of anemia, include antidepressants, psychostimulants (such as methylphenidate), and exercise.40,41
TREATMENT OF ANEMIA
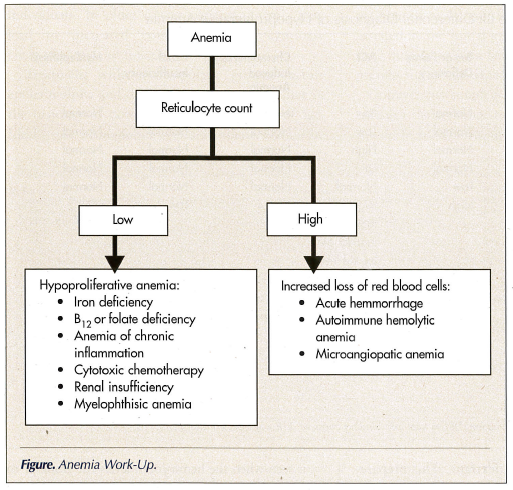
The treatment of anemia is a mainstay in the management of all cancer patients. The treatment must be based on the specific cause of anemia. The work-up of anemia is illustrated in the Figure. The reticulocyte counts indicate whether anemia is due to increased loss or decreased production of erythrocytes. In the majority of cases, the anemia of cancer is hypoproliferative. Causes of increased loss of red blood cells include hemolytic anemia and acute hemorrhages. Hemolytic anemia may be autoimmune (lymphoproliferative disorders) or microangiopathic, from disseminated intravascular coagulation or neoangiogenesis. In addition, cancer may be a cause of vasculitis.
In the case of hypoproliferative anemia, it is important to rule out iron, B12, or folate deficiency, anemia of chronic inflammation (ACI), and chemotherapy-induced anemia, as well as myelophthisis and renal insufficiency. The differential diagnosis is illustrated in Table III.
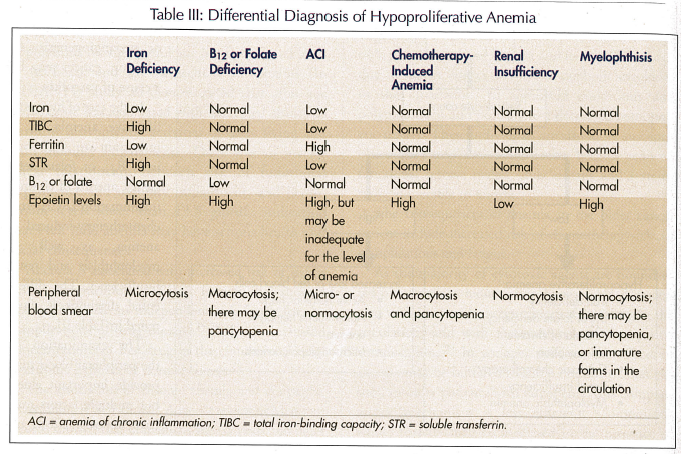
The examination of the peripheral blood may provide important clues, but multiple factors may have opposite effects on the size of the red blood cells.3 For example, iron deficiency causes microcytosis, cytotoxic chemotherapy macrocytosis, and the summation of both conditions, normocytic anemia.
Generally, iron deficiency and ACI may be differentiated on the basis of iron, total iron-binding capacity (TIBC), and ferritin levels. When these results are confusing, soluble transferrin receptor levels generally provide the proper diagnosis. Epoietin levels are low in anemia of renal insufficiency; in anemia of chronic inflammation they may be inappropriately low for the levels of anemia. Bone marrow examination is necessary for the diagnosis of myelophthisis, but otherwise it may be avoided.
In the case of iron deficiency, the treatment consists of elimination of the cause of blood loss, when feasible, and iron replacement. Oral iron replacement may not be effective, due to poor compliance with the medication that causes nausea, constipation, gastric irritation, and poor absorption. Several parenteral iron preparations are available. One may replace the full iron reserve with a single dose of iron dextrane. This preparation is inexpensive but, rarely, it may cause anaphylactoid reactions. Iron gluconate may only be administered in small, repetitive doses, and iron sucrose is very expensive.
Most patients with B12 deficiency respond to oral administration of crystalline B12. Parenteral B12 assures compliance and absorption, and it can be administered in any doctor’s office.
Epoietin and darbepoietin are the mainstay treatment of ACI, chemotherapy-induced anemia, and anemia from renal insufficiency. Doses for ACI and chemotherapy-induced anemia are higher, due to a relative resistance to erythropoietin in these conditions.37-39,42 A number of studies in cancer patients of all ages have demonstrated that:
• Treatment of anemia with erythropoietic growth factors leads to improvement of energy levels and quality of life.
• Energy and quality of life increase linearly with the increase in hemoglobin, and the highest increment is seen when the hemoglobin levels rise from 11 to 13 gm/dL.
• Age does not influence the response to epoietin or darbepoietin, nor the degree of improvement in quality of life and energy levels.
Until recently, it has been debated whether treatment with epoietin should have been supplemented with iron. The rationale for using iron is that the iron stored in the bone marrow of patients with ACI is not easily mobilized, due to hepcidin and catabolic cytokines.
In a recent randomized, controlled study, Auerbach et al43 demonstrated that intravenous supplementation of iron doubled the response to erythropoietin and was significantly superior to oral iron supplementation.43
CONCLUSIONS
Anemia—even mild anemia—has a negative influence on the survival, function, and health of older individuals. In older cancer patients, anemia may lead to increased complications of cytotoxic chemotherapy and functional dependence.
Treatment with erythropoietic growth factors epoietin-alpha and darbepoietin-alpha is effective in the majority of cancer patients, irrespective of age. Ideally, the hemoglobin of older patients should be maintained at 12 gm/dL. The treatment should be instated at the initial symptom of fatigue and anytime the hemoglobin levels drop below 10 gm/dL to prevent functional dependence.
Intravenous supplementation of iron appears to enhance the response to erythropoietic agents, and should be used concomitantly with these agents.










