ADVERTISEMENT
Diagnostic Challenges and Treatment Options: Case Presentation on Cognitive Decline and Dementia
THE CASE
A 75-year-old female is brought in by her two daughters with complaints of short-term memory loss of 2 years duration. They now agree to move her to an assisted living center. She had been independent up to the time of her stroke 2 years ago, and ever since it has been a “struggle.” There has been a loss of interest in daily activities and there has been a decline in concentration. The woman has no motor deficits from the stroke but has become a bit more suspicious of at least one daughter. The eldest child believes that nothing is wrong and that the changes observed just occur with aging. The younger daughter spends much more time with her mother and thinks there is something more serious going on. The patient’s history includes treated hypertension, for which she takes medication regularly. She has no history of diabetes, has never smoked, has no known coronary artery disease, and despite what appears to be apathy, has not lost any weight. Her husband died 5 years ago, and her two older sisters are reported to have Alzheimer’s disease. The patient has no other children besides the two daughters.
BACKGROUND
 The Alzheimer’s Association has published its 10 warning signs (Table I). Each warning sign taken in isolation can be an extension of what we can expect from the normal aging process. However, taken together or in the context of a change from one’s former level of intellectual function, they can be the earliest signs of disease.
The Alzheimer’s Association has published its 10 warning signs (Table I). Each warning sign taken in isolation can be an extension of what we can expect from the normal aging process. However, taken together or in the context of a change from one’s former level of intellectual function, they can be the earliest signs of disease.
An estimated 4.5 million Americans now have Alzheimer’s disease, and that number has more than doubled since 1980. The number of Americans with Alzheimer’s disease will continue to grow, and by 2050 the number of affected persons could range between 11.3 to 16 million adults.1 Current thinking holds that Alzheimer’s disease remains the most common dementia. However, the impact of vascular disease is significant and likely attributes to well over 40% of all dementing illnesses.2
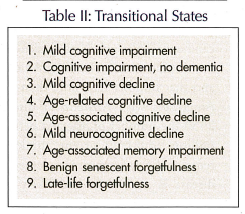 Particular interest has been placed on the transitional states. These have gone by a variety of different names (Table II). We remain most interested in the concept of mild cognitive impairment (MCI), and a variety of trials have been undertaken with patients having MCI to determine the probable progression to Alzheimer’s disease. The most important recently completed trial was conducted by the Alzheimer’s Disease Centers Cooperative Group. Petersen et al3 have already reported the preliminary findings at the July 2004 World Congress on Alzheimer’s Disease. The study, “Donepezil and Vitamin E in the Treatment of Mild Cognitive Impairment,” showed no benefit from vitamin E in reducing the incidence of Alzheimer’s disease. Donepezil delayed the appearance of Alzheimer’s disease, but only for the first 18 months of the study. Beyond that time point, placebo- and donepezil-treated patients with MCI showed the same rate of development of Alzheimer’s disease.
Particular interest has been placed on the transitional states. These have gone by a variety of different names (Table II). We remain most interested in the concept of mild cognitive impairment (MCI), and a variety of trials have been undertaken with patients having MCI to determine the probable progression to Alzheimer’s disease. The most important recently completed trial was conducted by the Alzheimer’s Disease Centers Cooperative Group. Petersen et al3 have already reported the preliminary findings at the July 2004 World Congress on Alzheimer’s Disease. The study, “Donepezil and Vitamin E in the Treatment of Mild Cognitive Impairment,” showed no benefit from vitamin E in reducing the incidence of Alzheimer’s disease. Donepezil delayed the appearance of Alzheimer’s disease, but only for the first 18 months of the study. Beyond that time point, placebo- and donepezil-treated patients with MCI showed the same rate of development of Alzheimer’s disease.
The progression of Alzheimer’s disease is slow and relentless, usually taking 8-10 years to fully manifest itself in the typical patient in their late 70s or early 80s. In the mild stages of the disease, cognitive problems predominate. These include memory loss, language problems, mood swings, personality changes, diminished judgment, and impaired orientation. As the disease progresses into its moderate or middle stages, functional difficulties become more apparent. There can be changes in personality and behavior, there is the inability to learn and recall new information, and long-term memory is affected. There is difficulty performing both basic and instrumental activities of daily living. It is at the middle stages of the disease that patients usually transition from home to some type of institutional setting.
In the severe or late stage of the disease, behavioral problems are most manifest. These include agitation, paranoia, and confusion. There are gait disturbances, falls, incontinence, and motor disturbance. There is loss of language and complete inability to perform even the most basic activities of daily living (ADLs).
Cholinesterase inhibitor therapy has been utilized since the release of tacrine nearly 11 years ago. Three drugs remain active in the treatment of Alzheimer’s disease based on the cholinergic hypothesis; they include donepezil, galantamine, and rivastigmine. The reversible cholinesterase inhibitors include donepezil, galantamine, and tacrine, while the pseudo-irreversible inhibitor on the market is rivastigmine. These drugs were approved for mild-to-moderate disease but have shown benefit in treating cognitive, functional, and behavioral manifestations of disease. The N-methyl-D-aspartate antagonist memantine was approved by the Food and Drug Administration in 2003. It has been used for over 20 years in Europe and is now available in the United States for moderate-to-advanced Alzheimer’s disease.
The relationship between hypertension and Alzheimer’s disease continues to be elucidated. Hypertension appears to be a strong risk factor for vascular dementia and possibly for Alzheimer’s disease. The risk for dementia increased in Japanese Americans with higher blood pressure, as noted in the Honolulu Heart Study.4 Others have shown that hypertension almost doubles the risk of vascular dementia, and that hypertension with heart disease is associated with a threefold increase in the risk of vascular dementia. Hypertension with diabetes is also associated with a sixfold increase in the risk of vascular dementia.5 The hypothesized pathologic effects of hypertension include vascular remodeling, impairment of cerebral autoregulation, cerebral microbleeds, white matter lesions, unrecognized lacunar infarcts, and Alzheimer-like changes, including cerebral atrophy and amyloid angiopathy.6
The Epidemiology of Vascular Aging (EVA) study showed that there was a higher risk of cognitive decline in untreated patients with hypertension when compared to treated patients.7 The Kungsholmen Project also showed protective benefits of diuretics against dementia,8 and the Heart Outcomes Prevention Evaluation (HOPE) study showed that long-term antihypertensive treatment may reverse cognitive impairment.9 In the Vascular Dementia Project, which was a subset of the Systolic Hypertension in Europe (Syst-Eur) trial,10 after median follow-up of 2 years, the incidence of dementia was reduced in 50% of treated patients. The benefit of treating hypertension in 1000 patients for 5 years is that it could prevent 20 cases of dementia.10
There has been relatively little work on risk factors for clinically defined MCI, but there is a growing literature on the overlap of cardiovascular risk factors for both Alzheimer’s disease and vascular dementia.11-13 These same risk factors are also associated with cognitive decline in older adults without dementia.14 Some of the major vascular risk factors for cognitive decline and dementia include hypertension, cardiovascular disease, diabetes, elevated serum homocysteine, and apolipoprotein E genotype. To the extent that MCI as a clinical diagnosis falls logically in between cognitive decline and dementia, it is reasonable to assume that these same risk factors are operative on MCI as well.
Drugs currently used for the treatment of Alzheimer’s disease only improve symptoms; they do not cure or even arrest the progression of disease. Acetylcholinesterase inhibitors may improve, maintain, or slow the decline of cognitive, behavioral, and functional performance in patients with mild-to-moderate disease. A variety of studies have consistently shown an effect in all three domains. Effects may be modest, but are consistently better than placebo, especially regarding cognitive effects.15 The deterioration of ADL function is representative of drug effect on the middle stages of disease. Donepezil has had some significant impact in this regard.16 Additionally, galantamine has been shown to have an effect on late-stage disease and it is presumed that all three drugs in the cholinesterase inhibitor family have effects on cognition, function, and behavior.17
Many of the current strategies to preserve cognitive function relate to general well-being and an overall control of multiple comorbidities. The Alzheimer’s Association’s Maintain Your Brain campaign is built along these lines. Hypertension occurs in greater than 30% of the elderly, and diabetes mellitus occurs in 10%. Treatment of these common cerebrovascular risk factors is available now and should have benefits at both the individual patient level as well as the population level for reducing the burden of cognitive impairment and dementia.
Cholinesterase drug therapy has had a positive impact on cognition and function in vascular dementia.18 Galantamine has had a positive effect on daily function in patients with Alzheimer’s disease who have cerebrovascular disease or vascular dementia.19 With the addition of memantine, a glutamate receptor antagonist, physicians have the opportunity for additive therapy to provide additional symptomatic relief for patients with moderate-to-advanced disease. Memantine and donepezil in combination were shown to be more effective than donepezil plus placebo in observing mean change from baseline along the severe impairment battery.20
In a double-blind, randomized trial evaluating the direct medical cost and caregiver cost by including donepezil therapy, there was reduced caregiver time by 400 hours over a 1-year period. Total costs were $1100 less for patients receiving donepezil than for those not receiving donepezil.21 Another study employing rivastigmine treatment also measured the caregiver hours saved in treating patients with mild disease. The hours saved were 691 with a value assigned of $8808 per patient. For those with moderate disease, the hours saved were 204 hours at a value of $2008.22 Again, supporting a likely class effect from these interventions, Sano and colleagues23 showed that the effect of galantamine on caregiver time spent assisting with ADLs was also improved using drug therapy rather than placebo.
Given the tremendous expense in terms of decline in function and cost to the health care system, patients benefit more from residing in their households than transferring to assisted living facilities or nursing homes. The impact of cholinesterase inhibitor treatment on nursing home placement remains controversial. The time to first dementia-related nursing home placement with increasing donepezil exposure was also shown to be improved.24 The best improvement occurred when the drug therapy was at its maximal level and when it was applied as early in the diagnosis as possible. The cost effectiveness of rivastigmine and probability of institutionalization has been calculated by Hauber et al.25 Given a projected decline and Mini-Mental State Examination with and without therapy over a 2-year time horizon, the estimated cost savings of $4800 over a 2-year period of time can be attributed to patients with mild Alzheimer’s disease. The impact of donepezil on annual cost in a managed care plan has also been studied. Annual cost savings of donepezil versus no donepezil are presumed to be $3891, and again, the best savings are achieved with long-term use of therapy versus short-term use of therapy.26 However, a recent study on the cost effectiveness of donepezil found no long-term benefit and created quite a controversy over the study design, which included a washout period and a recruitment of only one-fifth of the intended population.27
The cost effectiveness of memantine used alone has also been calculated by Wimo and associates.28 As part of a clinical trial of memantine for moderate-to-severe disease, data on the costs of health care, caregiving, and lost productivity were used. Memantine was calculated to reduce cost by $1090 per month, with the greatest impact on direct caregiver costs. Needless to say, much remains to be done in valuing drug therapy across populations and different economic systems and priorities across nations.
TREATMENT OPTIONS
The patient in the aforementioned case study had a dementing illness. A vascular component most probably exists. An evaluation that would include an assessment of her general health as well as neuropsychological tests would be beneficial. A head scan would certainly help clarify the vascular component, and magnetic resonance imaging studies are very much a routine part of an evaluation where uncertainty exists.
Drug therapy can be offered once a better understanding of the underlying disease is apparent. For the most part, cholinesterase inhibitors find their way into the treatment of the vascular dementias because there is likely so much overlap with the degenerative dementias. These drugs also tend to improve attention and concentration, perfect attributes when experiencing a new environment, such as the proposed move to assisted living. Both of the patient’s daughters can be helped by having a better understanding of the disease. The patient would benefit from earlier intervention, including both drug and non-drug therapy to preserve function and maintain independence.
SUMMARY
The opportunities to treat disease include a variety of symptomatic therapies. Given the striking vascular component to dementia, blood pressure control and management of cholesterol are important to all patients with Alzheimer’s disease. The sooner disease is recognized and addressed, the greater the benefit. Studies report benefit on cognition, function, and behavior, so even later in the course of disease drug therapy remains an option. The most important aspect of the geriatrics practitioner may be the positive impact therapy has on families. This includes both direct and indirect caregiving costs. Though no cure or prevention yet exists, minimizing symptomatic disease manifestations benefits patients, families, and society.
The research reported in this article was supported by National Institutes of Health grants P50 AG16574 and V01 AG06786. Dr. Tangalos reported that he has served as a consultant for Eli Lilly and Company, Abbott Laboratories, Janssen Pharmaceutica, and Forest Laboratories, Inc.










