Using Active Leptospermum Honey to Address Transcutaneous Driveline Infection
Left ventricular assist devices (LVADs) are implanted into patients with severe heart failure. These mechanical circulatory support devices attach to the left ventricle and pump blood to the aorta. Although the devices have become smaller as technology advances, they still require transcutaneous drivelines to supply power to the pump. The driveline exits the patient’s left or right abdomen. Infectious complications are common in LVAD patients, occurring in up to 28% of patients within 3 months of implantation.1,2 Infections may be local at the driveline site or systemic, occur at any time during the patient support period (up to five or more years), and result in overwhelming sepsis and subsequent mortality; they significantly increase the cost of LVAD therapy.3 Infections, particularly those associated with malodorous and/or highly exudating wounds, may negatively affect patient quality of life. Early eradication of infection is of paramount importance.
Active Leptospermum honey (ALH) has been demonstrated effective in eradicating organisms often responsible for LVAD infections, including Burkholderia cepacia, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Candida spp., Acinetobacter spp., vancomycin-resistant Enterococcus, methicillin-resistant S. aureus, and multiresistant Gram-negative organisms.4,5 The product is able to reduce both wound odor (due to the honey’s ability to release hydrogen peroxide) and wound exudate (due to the osmotic activity of the high sugar content) and to inhibit bacterial growth because of its acidic pH.6 No pathogen resistance to the antibacterial effects or serious adverse effects have been reported with honey use.
A protocol using sterile dressings impregnated with ALH was initiated on a patient who presented with suppurative chronic driveline infections nonresponsive to intravenous antibiotic therapy and standard wound care.
Case Study
A 37-year-old African American man with an LVAD implant for end-stage heart failure due to non-ischemic cardiomyopathy presented on postoperative day 740. His comorbid conditions included morbid obesity, hyperlipidemia, osteoarthritis, hepatitis C, renal failure, and depression. During LVAD support, he experienced recurrent bloodstream and driveline infections with a variety of Gram-positive (mostly S. aureus) and Gram-negative (Klebsiella, Pseudomonas) organisms. It was assumed that the abdominal pocket housing the LVAD was chronically infected. Antibiotic therapy options were limited by the renal and hepatic pathologies. The definitive treatment required was heart transplantation and removal of the LVAD.
The driveline wound had significant, malodorous exudate (see Figure 1) and required intermittent hospitalizations for intravenous antibiotic therapy. As a last resort, treatment with ALH-impregnated alginate (Medihoney®, Derma Sciences, Princeton, NJ) was initiated. Dressings were applied around the driveline and covered with gauze and tape. Dressing change frequency was daily at first, then every other day once drainage reduced. Reduction of odor and exudate was noted almost immediately (within 4 days), increasing patient comfort. Wound cultures continued to grow Pseudomonas organisms. After 18 days of treatment, ALH treatment of the driveline was aborted by cardiac transplant and removal of the driveline. The driveline site was noted to have only trace exudate, pink wound bed, and no odor at this time (see Figure 2). As suspected, at transplant the abdominal pump pocket was noted to be the primary site of infection. The pump pocket wound was irrigated and drained and vacuum-assisted closure dressing was applied. Wound healing was eventually achieved, almost certainly secondary to the removal of the infected device.
Considering the abdominal and device infections, it is unlikely that any topical wound treatment could have cleared the transcutaneous driveline site infection. However treatment with ALH reduced odor and exudate with no adverse effects, which led to increased patient comfort. It is unclear whether clear wound cultures could have been achieved with a longer duration of ALH therapy; however in future, ALH dressings may be used earlier in an attempt to avoid the device pocket involvement. 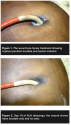
Making Progress With Stalled Wounds is made possible through the support of Derma Sciences, Inc., Princeton, NJ. The opinions and statements of the clinicians contained herein are specific to the respective authors and are not necessarily those of Derma Sciences, Inc., OWM, or HMP Communications.
This article was not subject to the Ostomy Wound Management peer-review process.
1. Holman WL, Rayburn BK, McGiffin DC, Foley BA. Infection in ventricular assist devices: prevention and treatment. Ann Thorac Surg. 2003;75:S48–S57.
2. Schulman AR, Martens TP, Christos PJ, et al. Comparisons of infection complications between continuous flow and pulsatile flow left ventricular assist devices. J Thorac Cardiovasc Surg. 2007;133:841–842.
3. Chinn R, Dembitsky W, Eaton L, Chillcott S, Stahovich M, Rasmusson B, Pagani F. Multicenter experience: prevention and management of left ventricular assist device infections. ASAIO J. 2005;51(4):461–470.
4. Blair SE, Cokcetin NN, Harry EJ, Carter DA. The unusual antibacterial activity of medical-grade Leptospermum honey: antibacterial spectrum, resistance and transcriptome analysis. Eur J Clin Microbiol Infect Dis. 2002;28(10):1199–1208.
5. Molan PC. The antibacterial activity of honey: the nature of the antibacterial activity. Bee World. 1992;73(1):59–76.
6. Lusby PE. Honey: a potent agent for wound healing? J WOCN. 2002;29:295–300.













