Use of Negative Pressure Therapy on Closed Surgical Incisions: A Case Series
Abstract
Multiple patient comorbidities and environmental factors increase the risk of incisional wound complications. The literature suggests that negative pressure therapy (NPT) on clean closed surgical incisions may help reduce the risk of wound infections and other complications. In this case study, NPT was applied in the operating room to clean closed surgical wounds in four high-risk patients (two men, two women) following coronary artery bypass grafting using bilateral internal mammary arteries, transmetatarsal amputation, and abdominal hysterectomy. All wounds healed well. These results and currently available information suggest that prospective, randomized, controlled clinical studies to assess the safety, efficacy, and cost-effectiveness of NPT in the prevention of postoperative wound complications are warranted. In addition, if studies confirm the validity and reliability of the proposed patient grading system discussed, it may help guide use of NPT in postsurgical patients.
Potential Conflicts of Interest: This article was prepared with the financial support of KCI, San Antonio, TX.
Please address correspondence to: Christopher E. Attinger, MD, The Georgetown Limb Center, Georgetown University Hospital, 3800 Reservoir Road, Washington, DC 20007; email: cattinger@aol.com.
Certain surgical incisions and patient conditions may adversely affect optimal wound healing, which could lead to postoperative complications. For example, the rate of complications such as wound necrosis and infection for high-energy trauma wounds can range from 33% to 50%.1 For postcardiothoracic surgery, sternal wound infection remains the most dreaded complication, resulting in 1-year mortality rates of 33% from mediastinitis after coronary artery bypass and subsequent reduced long-term survival.2,3 Patients with multiple comorbidities such as obesity, diabetes, and poor vascular status have been found to be at higher risk of complications (eg, infection, seroma, hematoma, dehiscence) associated with primary or delayed primary intention postoperative wound healing.4-6 Other primarily closed incisional wounds with a high risk of complications include wounds from hip and knee arthroplasty7; traumatic wounds with pilon, tibial plateau, and calcaneus fractures1,8; and wounds from lower-extremity bypass,1 abdominal,9,10 and cardiothoracic procedures.11
Surgical incisional wounds have traditionally been closed by primary intention using sutures, staples, adhesives, or a combination thereof. Traditional closure methods that include tensioning devices such as sutures and staples concentrate the spreading force of a large wound to small points along the wound and it has been suggested that this may result in ischemia and, possibly, necrosis of wound tissue.9
Following primary closure of clean surgical incisions, wound care may include the application of traditional gauze dressings10 and advanced therapies such as hydrocolloids,10 growth factors,12 cultured skin,13 low energy ultrasound,14 and negative pressure therapy (NPT).1,8,12 Advanced therapies such as topically applied growth factors,12 cultured skin,13 and NPT1,8,12,15,16 were developed to facilitate healing of difficult-to-heal chronic and acute wounds. NPT has become a key component of many wound treatment protocols. This therapy combines the use of a NPT device and device-specific dressings (medical grade reticulated open-cell foam — KCI Licensing Inc, San Antonio, TX) to create a negative pressure environment at the wound site. The described mechanisms of action for NPT include protecting the wound bed, splinting soft tissue, reducing edema,17 increasing perfusion,18-21 and enhancing development of granulation tissue.15,16
To enhance knowledge and guide decisions regarding which clean closed surgical wounds would benefit from this therapy, a review of the current literature on the use of NPT as an effective therapy for surgical incisions is presented, along with case reports of patients who received NPT therapy and a proposed grading system to assess the feasibility of NPT use in particular patients.
Negative Pressure Therapy and Surgical Incisions
A PubMed search of literature was conducted on the use of negative pressure wound therapy, negative pressure, and subatmospheric pressure on clean, closed surgical incisions. NPT has been studied in a variety of postsurgical wound types and used for wounds healing by both primary intention and delayed primary closure. The effects of NPT on surgical incisions have been reported in one randomized prospective clinical trial by Stannard et al,1,8 one retrospective study by Atkins et al,11 and a case series by Gomoll et al7 (see Table 1). NPT may help reduce the risk of wound infections and other complications.
 Stannard et al’s8 study compared the effect of NPT at -125 mm Hg to standard postoperative dressings used over closed incisions following high-energy trauma. The study sample comprised 262 patients with 273 calcaneus, pilon, and tibial plateau fractures. Of those, 141 were randomized to NPT and 121 were randomized to control treatment. The incidence of wound dehiscence and infections was found to be lower in the NPT compared to the control group. Wounds of 12 patients in the NPT and 21 in the control group (P <0.03) dehisced; wounds of 14 patients in the NPT and 24 in the control group became infected (P <0.02). The authors recommended that based on these results, NPT should be considered for high-risk wounds following severe skeletal trauma.
Stannard et al’s8 study compared the effect of NPT at -125 mm Hg to standard postoperative dressings used over closed incisions following high-energy trauma. The study sample comprised 262 patients with 273 calcaneus, pilon, and tibial plateau fractures. Of those, 141 were randomized to NPT and 121 were randomized to control treatment. The incidence of wound dehiscence and infections was found to be lower in the NPT compared to the control group. Wounds of 12 patients in the NPT and 21 in the control group (P <0.03) dehisced; wounds of 14 patients in the NPT and 24 in the control group became infected (P <0.02). The authors recommended that based on these results, NPT should be considered for high-risk wounds following severe skeletal trauma.
Atkins et al11 reported the results of a retrospective review using NPT at -125 mm Hg for 4 days on 57 adult patients who were at high risk for sternal wound infections after sternotomy. During that same time period, wounds of 213 patients not at risk for infection were managed with standard postoperative wound care. No complications were observed in the NPT group and the wound of one patient in the control group became infected. The author noted that the expected, literature-based, sternotomy infection complication rate for high-risk patients is 6.1% ± 4%.22 The authors concluded that NPT was an easily applied and well tolerated therapy and that strong consideration should be given to NPT as a form of “well wound” therapy for patients at increased risk for sternal wound infections.
Gomoll7 reported on a 35-patient case series of high-risk patients following revision hip arthroplasty, proximal femoral and tibial fracture fixation, and foot and ankle trauma surgery. NPT was applied at -75 mm Hg with a custom-made nonadhesive permeable dressing covered with 1˝ strips of reticulated open-cell polyurethane foam as an immediate postoperative dressing for an average of slightly more than 3 days. No wound infections occurred after a minimum follow-up of 3 months.
Patient Grading System
A universal patient grading system (PGS) is needed to determine which closed surgical incisions are best suited for NPT. Although not all surgical patients are candidates for NPT,  patients found to benefit from NPT are those at the greatest risk for infection, seroma, hematoma, and dehiscence because they have one or more comorbidities that may affect proper wound healing.4-6 For example, patients without pre-existing medical conditions known to affect healing may not be candidates for NPT as an adjunct to surgical incision closure by primary intention because their wounds usually heal well on their own. However, surgical patients with a number of known risk factors (eg, diabetes, obesity, and a history of smoking, hypertension, steroid use, and/or radiation exposure) may be suitable candidates for a therapeutic system that enhances the healing of surgically closed incisional wounds. A PGS could be helpful in determining which candidates are suitable for NPT (see Figure 1).
patients found to benefit from NPT are those at the greatest risk for infection, seroma, hematoma, and dehiscence because they have one or more comorbidities that may affect proper wound healing.4-6 For example, patients without pre-existing medical conditions known to affect healing may not be candidates for NPT as an adjunct to surgical incision closure by primary intention because their wounds usually heal well on their own. However, surgical patients with a number of known risk factors (eg, diabetes, obesity, and a history of smoking, hypertension, steroid use, and/or radiation exposure) may be suitable candidates for a therapeutic system that enhances the healing of surgically closed incisional wounds. A PGS could be helpful in determining which candidates are suitable for NPT (see Figure 1).
This proposed PGS assessment tool, developed with input from the authors and information in the literature,4-6 may help identify patients with clean closed surgical wounds that may be at risk for complications such as infection, seroma, hematoma, or dehiscence. Grade 1 patients have linear or semilinear wounds and no pre-existing medical conditions and are considered at low or no risk for the development of postsurgical complications. Grade 2 patients have linear or semi-linear wounds and at least one moderate to high-risk factor, making them candidates for postsurgical NPT. Known patient risk factors or comorbidities include diabetes, obesity, smoking, hypertension, steroid use, radiation  exposure, and other factors affecting wound healing (see Table 2). Grade 3 patients have linear, semilinear, or complex wounds with undermining and one or more comorbidities. From the authors’ experience, wounds and surgical incisions that may benefit from the prophylactic use of NPT include patients who are at high-risk for developing postsurgical complications; contraindications for use of primary closure and NPT include ischemia and wound contamination (see Table 3). In these instances, delayed primary closure via split-thickness skin grafts or flaps should be considered. This PGS must be used cautiously and remains a proposed concept until validated.
exposure, and other factors affecting wound healing (see Table 2). Grade 3 patients have linear, semilinear, or complex wounds with undermining and one or more comorbidities. From the authors’ experience, wounds and surgical incisions that may benefit from the prophylactic use of NPT include patients who are at high-risk for developing postsurgical complications; contraindications for use of primary closure and NPT include ischemia and wound contamination (see Table 3). In these instances, delayed primary closure via split-thickness skin grafts or flaps should be considered. This PGS must be used cautiously and remains a proposed concept until validated.
Case Studies
The following case studies are examples of the prophylactic use of NPT over clean closed surgical wounds. Patients were selected to demonstrate the technique only; they were treated by the authors over the past few years.
 Case study 1: coronary artery bypass grafting (CABG) with bilateral internal mammary arteries as conduit. Sixty-one-year-old Mr. J (PGS Grade 3) had a medical history that included previous aortic valve replacement via sternotomy, obesity (body mass index [BMI] of 32), diabetes, and renal failure. He began experiencing increased symptoms of angina and heart failure. Cardiac catheterization showed two-vessel coronary artery disease not amenable to percutaneous coronary intervention.
Case study 1: coronary artery bypass grafting (CABG) with bilateral internal mammary arteries as conduit. Sixty-one-year-old Mr. J (PGS Grade 3) had a medical history that included previous aortic valve replacement via sternotomy, obesity (body mass index [BMI] of 32), diabetes, and renal failure. He began experiencing increased symptoms of angina and heart failure. Cardiac catheterization showed two-vessel coronary artery disease not amenable to percutaneous coronary intervention.
Coronary artery bypass grafting (CABG) using bilateral internal mammary arteries due to age and lack of another available conduit for grafting was performed via a redo median sternotomy incision. The skin incision extended from approximately 5 cm below the sternal notch to just below the xiphoid process. The depth of the subcutaneous fat was more than 20 mm. When the procedure was completed, the sternum was re-approximated using stainless steel wire sternal circlage. The pectoralis fascia was re-approximated with running absorbable suture. A subcutaneous suture was used to reduce dead space in the peristernal area. The skin was closed with surgical staples and NPT at -125 mm Hg, continuous setting, was instituted for a total of five postoperative days, with one dressing change performed on postoperative day 2 to inspect the wound and remove mediastinal chest tubes and epicardial pacing wires.
Based on physician experience, most cardiac surgical patients are hospitalized for an average of 4 days; however, Mr. J was discharged on postoperative day 12. The sternal wound was intact and well healed at discharge (see Figure 2a–d). 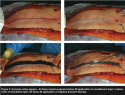
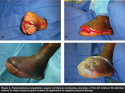
Case study 2: transmetatarsal amputation wound. Mr. K, 66 years old, presented with a painful chronic nonhealing ulceration of his left forefoot. Over the previous 4 years, he had undergone multiple debridements and partial left foot amputations to treat recurrent necrotic ulcerations. His medical history included diabetes, hypertension, and peripheral arterial disease (PGS Grade 3). At the time of presentation, Mr. K was noted to have a deeply probing ulcer, a prominent second metatarsal head, and persistent necrotic tissue. Given his multiple comorbidities and recurrent ulceration, osteomyelitis was a concern.
Mr. K was taken to the operating room and underwent initial debridement, with removal of all necrotic tissue (including bone) in preparation for a final stage closure. Cultures and tissue specimens were collected and sent to pathology, after which intravenous antibiotics were started. At the conclusion of the operation, Mr. K had an open transmetatarsal amputation and NPT was applied. Intraoperative cultures revealed Enterobacter cloacae and group B streptococcus. Mr. K was returned to the OR several days later for final debridement and closure. Once healthy viable tissue was evident, the transmetatarsal amputation was closed. Given his history of persistent wound breakdown and dehiscence, NPT at -125 mm Hg, continuous setting, was utilized on the closed surgical incision at the conclusion of surgery and continued for three postoperative days. Mr. K tolerated the procedure well and was discharged on postop day 3 with a healthy, intact incision. No complications, including seroma, dehiscense or infection, were noted. Mr. K has gone on to heal and he is able to ambulate (see Figure 3a–d).
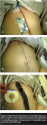
 Case study 3 and case study 4: abdominal hysterectomy. Two patients (PGS Grade 3) underwent a total abdominal hysterectomy with bilateral salpingo-oophorectomy and bilateral pelvic lymphadenectomy and bilateral para-aortic lymph node sampling. Ms. L (see Figure 4a–c) is a morbidly obese (BMI 50) woman in her 30s with diabetes and endometrial cancer; she had a previous C-section and hernia repair with mesh. Ms. M (see Figure 5a-c) is a morbidly obese (BMI 60) woman, also in her 30s with endometrial cancer. She has hypothyroidism and no history of prior surgeries.
Case study 3 and case study 4: abdominal hysterectomy. Two patients (PGS Grade 3) underwent a total abdominal hysterectomy with bilateral salpingo-oophorectomy and bilateral pelvic lymphadenectomy and bilateral para-aortic lymph node sampling. Ms. L (see Figure 4a–c) is a morbidly obese (BMI 50) woman in her 30s with diabetes and endometrial cancer; she had a previous C-section and hernia repair with mesh. Ms. M (see Figure 5a-c) is a morbidly obese (BMI 60) woman, also in her 30s with endometrial cancer. She has hypothyroidism and no history of prior surgeries.
Both patients had end bloc resection of a pelvic tumor and rectosigmoid resection, with low re-anastomosis and protective ileostomy. Their incisions measured 30 cm to 40 cm in length and 10 cm to 12 cm in depth. Adipose closure was re-approximated, the skin closed with staples, and NPT applied over the incision under sterile conditions in the OR. The NPT drape was cut into strips and  positioned on both sides of the staple line, with any dips or creases filled with stoma paste. Either Adaptic (Johnson & Johnson, Piscataway, NJ) or Mepitel (Mölnlycke Health Care, Norcross, GA) was applied over staples, covered with reticulated open-cell polyurethane black foam (Granufoam, KCI Licensing, San Antonio, TX), and secured with an adhesive drape. NPT was applied at -125 mm Hg continuous pressure for 4 days.
positioned on both sides of the staple line, with any dips or creases filled with stoma paste. Either Adaptic (Johnson & Johnson, Piscataway, NJ) or Mepitel (Mölnlycke Health Care, Norcross, GA) was applied over staples, covered with reticulated open-cell polyurethane black foam (Granufoam, KCI Licensing, San Antonio, TX), and secured with an adhesive drape. NPT was applied at -125 mm Hg continuous pressure for 4 days.
Total NPT duration for both patients was 4 days, with no reapplication of dressing required. Ms. L was discharged home from an acute hospital setting. Superficial skin separation of 3 mm to 5 mm was documented on postoperative day 14 after staple removal. A local dressing was applied, and the incision completely healed in about 2 weeks. Ms. M was discharged home from an acute hospital setting and the wound healed well in 4 days.
Discussion
This review and case reports address the use of NPT to help prevent skin breakdown and promote a wound-healing environment for surgically clean, closed incisional wounds.
 Potential and observed benefits associated with the NPT mechanisms of action include providing a closed wound environment, reducing edema, increasing perfusion, removing infectious materials, and splinting the incision.15-21 The authors also note that placement of the NPT system in the sterile environment of the operating room at the time of closure combined with a subsequent low frequency of dressing changes may contribute to fewer infections. This is supported by the observations of Gomoll et al,7 who stated that the practical benefits of fewer dressing changes during the immediate postoperative period using NPT may help facilitate a cleaner wound environment.
Potential and observed benefits associated with the NPT mechanisms of action include providing a closed wound environment, reducing edema, increasing perfusion, removing infectious materials, and splinting the incision.15-21 The authors also note that placement of the NPT system in the sterile environment of the operating room at the time of closure combined with a subsequent low frequency of dressing changes may contribute to fewer infections. This is supported by the observations of Gomoll et al,7 who stated that the practical benefits of fewer dressing changes during the immediate postoperative period using NPT may help facilitate a cleaner wound environment.
Using propensity scores established by Fowler et al,22 Mr. J (the first case study), had a pre-operative risk of approximately 20% for deep sternal wound infection based on his comorbidities and the requirement for harvesting bilateral internal mammary arteries to complete the CABG. Atkins et al11 demonstrated that NPT use on clean, closed sternotomy incisions in patients at high-risk for infection resulted in no complications, including no sternal wound infections. Also, NPT has been shown in animal studies23,24 (n = 20 and n = 6, respectively), to have improved sternal perfusion in different states of sternal vascularity.
Additionally, Stannard et al8 reported a significant reduction of wound dehiscence and infection in high-risk fracture patients when NPT was applied to their surgical incisions following closure. Stannard et al’s8 interpretation of the data supports the notion that NPT may be used effectively as a prophylactic treatment to prevent wound dehiscence and infection in high-risk surgical incisions following severe skeletal trauma. In patients at risk for wound healing who have experienced previous wound breakdown, as was the situation in the second case study, NPT may help prevent breakdown following surgery. The very high-risk abdominal wound patients (PGS Grade 3) described in Cases 3 and 4 healed well with only very superficial wound separation documented in one of the patients and no outpatient NPT. In this study, the PGS was used retrospectively to describe conditions in each case; therefore, the PGS had no effect on treatment selection. The PGS needs to be validated in a large, prospective case series or randomized clinical controlled trial.
In addition, Stannard et al’s1 and Atkins et al’s11 use -125 mm Hg in their practices. The case studies presented herein indicate pressure setting based on clinician experience.
Limitations
Although the published works cited in this manuscript show a trend toward the successful use of NPT in clean closed surgical incisions, the limitations of this review include a lack of published clinical studies and the fact that the majority of research has been conducted utilizing products produced by a single NPT manufacturer at one designated negative pressure. Prospective, randomized controlled clinical studies to validate the safety, efficacy, and cost- effectiveness of NPT on clean closed surgical incisions are needed. Specifically, controlled clinical studies are required to confirm that this therapy reduces the rate of infection and other wound complications.
Conclusion
NPT has been shown to be an effective treatment for acute and chronic open wounds. This review describes existing literature about the use of NPT in the management of closed clean surgical incisions and results of its application in four patients at risk for wound complications. Results suggest that at-risk patients may benefit from this treatment during the immediate postoperative period. For optimal practicality, the NPT system should have an easy-to-apply dressing and a lightweight NPT device that is readily available in the OR, easy to use, and portable to allow for patient mobility in both the inpatient and outpatient settings. Due to the lack of published evidence, additional studies are needed across the various surgical incision types to assess the safety, effectiveness, and cost-effectiveness of prophylactic NPT use.
Acknowledgment;
The authors thank Stephanie Wasek and Ricardo R. Martinez for editorial and manuscript preparation and assistance.
1. Stannard JP, Robinson JT, Anderson ER, McGwin G Jr, Volgas MD, Alonso JE. Negative pressure wound therapy to treat hematomas and surgical incisions following high-energy trauma. J Trauma. 2006;60(6):1301–1306.
2. Hollenbeak CS, Murphy DM, Koenig S, Woodword RS, Dunagan WC, Fraser VJ. The clinical and economic impact of deep chest surgical site infections following coronary artery bypass graft surgery. Chest. 2000;118:397–402.
3. Karra R, McDermott L, Connelly S, Smith P, Sexton DJ, Kaye KS. Risk factors for 1-year mortality after postoperative mediastinitis. J Thorac Cardiovasc Surg. 2006;132:537–543.
4. Wilson JA, Clark JJ. Obesity: impediment to postsurgical wound healing. Adv Skin Wound Care. 2004;17(8):426–435.
5. Riou JPA, Cohen JR, Johnson H. Factors influencing wound dehiscence. Am J Surg. 1992;163:324–330.
6. Abbas SM, Hill AG. Smoking is a major risk factor for wound dehiscence after midline abdominal incision; case-control study. ANZ J Surg. 2009;79:247–250.
7. Gomoll AH, Lin A, Harris MB. Incisional vacuum-assisted closure therapy. J Orthop Trauma. 2006;20(10):705–709.
8. Stannard JP, Volgas DA, McGwin G, et al. Negative pressure wound therapy following high-risk lower extremity fractures. Poster presented at the Annual Meeting of the Orthopaedic Trauma Association. Boston, MA. October 2007.
9. Easterlin B, Bromberg W, Linscott J. A novel technique of vacuumassisted wound closure that functions as a delayed primary closure. Wounds. 2007;19(12):331–333.
10. Holm C, Petersen JS, Grønboeck F, Gottrup F. Effects of occlusive and conventional gauze dressings on incisional healing after abdominal operations. Eur J Surg. 1998;164(3):179–183.
11. Atkins BZ, Wooten MK, Kistler J, Hurley K, Hughes GC, Wolfe W. Does negative pressure wound therapy have a role in preventing poststernotomy wound complications? Surg Innov. 2009;May 21. [Epub ahead of print].
12. Wu L, Mustoe TA. Effect on ischemia on growth factor enhancement of incisional wound healing. Surgery. 1995;117(5):570–576.
13. Veves A, Falanga V, Armstrong DG. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001;24:290–295.
14. Ennis WJ, Formann P, Mozen N, Massey J, Conner-Kerr T, Meneses P. Ultrasound therapy for recalcitrant diabetic foot ulcers: results of a randomized, double-blind, controlled, multicenter study. Ostomy Wound Manage. 2005;51(8):24–39.
15. Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366:1704–1710.
16. Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers. Diabetes Care. 2008;31:631–636.
17. Yousaf M, Witherow A, Gardiner KR, Gilliland R. Use of vacuum-assisted closure for healing of a persistent perineal sinus following panproctocolectomy: report of a case. Dis Colon Rectum. 2004;47(8):1403-1407;discussion 1407–1408.
18. Argenta LC, Morykwas MJ, Dersch T. Effects of negative pressure on skin oxygen tension and perfusion. 4th Annual Meeting of the European Tissue Repair Society. Oxford, UK. August 25–28, 1994.
19. Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuumassisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38(6):553–562.
20. Wackenfors A, Sjögren J, Gustafsson R, et al. Effects of vacuum-assisted closure therapy on inguinal wound edge microvascular blood flow. Wound Repair Regen. 2004;12(6):600–606.
21. Ichioka S, Watanabe H, Sekiya N, Shibata M, Nakatsuka T. A technique to visualize wound bed microcirculation and the acute effect of negative pressure. Wound Repair Regen. 2008;16(3):460–465.
22. Fowler VS, O’Brien SM, Muhlbaier LH, Corey R, Ferguson TB, Peterson ED. Clinical predictors of major infections after cardiac surgery. Circulation. 2005;112(suppl I):I358–I365.
23. Wackenfors A, Gustafsson R, Sjogren J, Ingemansson R, Malmsjo M. Blood flow responses in the peristernal thoracic wall during vacuum- assisted closure therapy. Ann Thorac Surg. 2005;79:1724–1731.
24. Petzina R, Gustafasson L, Mokhtari A, Ingemansoon R, Malmsjo M. Effect of vacuum-assisted closure therapy on blood flow in the peristernal thoracic wall after internal mammary artery harvesting. Eur J Cardiothorac Surg. 2006;30:85–89.













