A Retrospective, Longitudinal Study to Evaluate Healing Lower Extremity Wounds in Patients with Diabetes Mellitus and Ischemia Using Standard Protocols of Care and Platelet-Rich Plasma Gel in a Japanese Wound Care Program
Abstract
Chronic wounds, especially in patients with diabetes mellitus (DM), are a major health challenge in Japan. The goal of wound care centers (WCCs) in Japan is to facilitate healing and prevent lower extremity amputations (LEAs) using standardized protocols of patient and wound care. The standard treatment algorithm includes a complete patient and wound assessment, history, physical exam, and a variety of diagnostic tests that determine the need for infection control intervention, revascularization, excision and debridement, growth factor/platelet rich plasma (PRP) gel therapy, skin graft/flap, wound protection, and education. All patient and wound data are entered in a secure central database for all WCCs. To evaluate the outcomes of standard care regimens compared to the use of a topical PRP gel treatment in patients with a variety of complex wounds, a retrospective, longitudinal study was conducted. Wound outcomes from 39 patients with 40 chronic, nonhealing, lower extremity wounds were evaluated between two time periods: between first presentation at the WCC (T1) and after using standard topical treatments (T2) and between T2 and after using the PRP gel treatment (T3). Patient average age was 66.8 years (SD: 10.60) and mean wound duration was 99.7 days before treatment (SD: 107.73); and the majority of patients (85%) had DM. Wounds were classified as ischemic diabetic (n = 24), diabetic (n = 10), ischemic (n = 5), and pressure ulcer (n = 1). DFUs were Wagner lll (77%) and lV (23%). Of those, 60% were in patients with arteriosclerotic obliterans (ASO). Infection (abscess, cellulitis, osteomyelitis, and/or gangrene) was present in all wounds and treated using debridement, antibiotic therapy, and surgery as deemed appropriate. During the first treatment period (T1 to T2) of 75.3 days, which included revascularization and/or debridement along with standard of care, none of the wounds healed and the average wound area, depth, and volume increased. Following topical PRP gel treatment, 83% of wounds healed within 145.2 days (T2 to T3) (P = 0.00002). Only one patient required an LEA. The results of this study suggest that good healing outcomes and a low amputation rate can be obtained with a protocol of supportive care (including revascularization procedures) and the PRP gel treatment. Prospective controlled studies comparing the use of this PRP gel to other advanced treatments are warranted.
Potential Conflicts of Interest: Funding for independent statistical analysis and manuscript preparation was provided by Cytomedix, Inc (Gaithersburg, MD). The authors disclosed no conflicts.
Introduction
National populations diagnosed with diabetes mellitus (DM) show China has the largest number of individuals with 90 million, the US ranks third with 23.7 million, and Japan ranks sixth with 10.7 million.1 A recent survey of 95.3 million Japanese citizens found an estimated 11.2% have been diagnosed with DM.1 Japanese government 2011 estimates2 anticipated that 3.6 million Japanese individuals ages 40 to 59 years and 6.5 million ages 60 to 79 years would be diagnosed with DM, an 11.2% estimated prevalence.
A Japanese government survey3 of 8,000 persons receiving care for their DM-related wounds suggests the problem may be under-reported. The study calculated that the number of individuals with an HbA1c >6.1% or who were getting DM treatments was 8.9 million, but that 13.2 million individuals were suspected to have DM because their HbA1c levels were between 5.6% and 6.1%. The study concluded that approximately 22.1 million individuals had or were suspected of having DM, which would be more than 18% of the Japanese population.
 Government agencies estimate the number of lower limb amputations secondary to DM are approximately 10,000/year (0.1%) in Japan and 66,000/year (0.3%) in the US.4,5 A review of the literature6 noted that approximately 20% of infected diabetic foot ulcers (DFU) ultimately lead to some degree of lower extremity amputation (LEA).6 Persons who undergo DM-related LEA are 40% to 50% likely to have another amputation within 2 years, per the American College of Foot and Ankle Surgeon guidelines.7 Similarly, another literature review8 showed persons with critical limb ischemia (CLI) and without DM who do not have successful revascularization will undergo LEA, have a 25% increase in mortality, and are 25% more likely to have an additional amputation within a year. Elucidating treatment methods to avoid LEAs in all patients is a quality-of-life issue as well as a financial challenge.
Government agencies estimate the number of lower limb amputations secondary to DM are approximately 10,000/year (0.1%) in Japan and 66,000/year (0.3%) in the US.4,5 A review of the literature6 noted that approximately 20% of infected diabetic foot ulcers (DFU) ultimately lead to some degree of lower extremity amputation (LEA).6 Persons who undergo DM-related LEA are 40% to 50% likely to have another amputation within 2 years, per the American College of Foot and Ankle Surgeon guidelines.7 Similarly, another literature review8 showed persons with critical limb ischemia (CLI) and without DM who do not have successful revascularization will undergo LEA, have a 25% increase in mortality, and are 25% more likely to have an additional amputation within a year. Elucidating treatment methods to avoid LEAs in all patients is a quality-of-life issue as well as a financial challenge.
The literature7 and national surveys9 indicate that the presence of peripheral arterial disease (PAD), also known as arteriosclerotic obliterans (ASO), can impact healing, is a predisposing factor for gangrene, and is a major risk factor for LEA in patients both with and without DM. A national health and nutrition survey9 found that persons with DM are twice as likely to develop ASO as persons without DM. The most severe form of ASO results in CLI; without intervention, prognosis for healing in patients who are unsuitable for revascularization is poor (30% amputation rate and 25% mortality rate at 1 year).10 Revascularization is typically attempted through use of peripheral angioplasty or endovascular or open surgical arterial bypass and is considered to be the primary treatment option.10 Treatment and wound healing outcomes for CLI patients are negatively affected by diabetes-related cardiovascular disease.10
Wounds on the lower extremity can be diagnosed as related to DM, ASO, pressure, venous, or of mixed etiology such as DM/ASO, or venous/ASO. A review of the literature11 has shown that in the US, the annual incidence of DFU is estimated to be 1.0% to 4.1%, but the lifetime incidence may be up to 25%. Although not as common as DFUs, 500 to 1,000 new ischemic ulcers per million patients (0.05% to 0.1%) are diagnosed yearly.10 Delayed healing and potential for infection are cause for concern in both DM and ischemic ulcers. Literature reviews6,12 have shown an estimated 56% of DFUs become infected, are a common reason for hospital admission, and lead to more than half of all nontraumatic LEAs. Similarly, a prospective study13 of 282 CLI ulcers found more than 58% were infected and/or gangrenous. The study also found that infection following revascularization diminished the likelihood of vascular patency (P <0.0005, OR 0.660); and presence of ulcer, gangrene, and infection were associated with increased odds of major LEA (P = 0.005, OR 34.626; P = 0.015, OR 17.358; P <0.0005, OR 0.927, respectively). These results indicate that the presence of an ulceration increased the odds of having a major LEA by 34.6.
The growing population of persons with DM, increased ASO incidence, and high incidence of amputations in Japan has led to the creation of specialty wound care centers (WCC) offering standardized wound care and use of consistent wound care algorithms.14 These WCCs focus on providing good wound care and a complementary revascularization strategy for CLI by involving surgical and cardiovascular departments in addition to a specialized wound care team and weekly scheduled telemedicine conferences to discuss each case. The goal of the WCCs is to reduce the rate of amputations and heal chronic wounds of all etiologies. The WCCs utilize a broad range of wound care therapies, including negative pressure wound therapy (NPWT) and platelet-rich plasma (PRP) gel for wounds that fail to respond to standardized care. Use of NPWT in chronic wounds has been shown clinically to substantially decrease wound size and reduce time to healing compared to standard wound care, making it a useful tool.15
In WCCs, PRP gel therapy (AutoloGel™ System, Cytomedix, Inc, Gaithersburg, MD; and Millennia Corporation, Tokyo, Japan) is used when standardized wound care is not healing the wound. The PRP gel therapy consists of plasma and platelets, yielding an autologous, near-physiologic 1.3 x baseline platelet concentration of growth factors, cytokines, and chemokines and a fibrin scaffold.16,17 As shown in an in vitro study,18 the platelet actively mediates wound healing by initiating the clotting cascade and releasing multiple growth factors such as platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), vascular endothelial cell growth factor (VEGF), platelet-derived angiogenic factor (PDAF), and transforming growth factor β (TGF b), all of which can influence the inflammatory phase.
Autologous PRP gel has been used clinically for more than two decades. Clinical trials have shown topically applying PRP gel to wounds not actively healing resulted in reductions in wound size and volume.17,19-21 In an RCT, comparative before-and-after design, and large case series, involving a total of 403 patients with DFUs, VLUs, and PUs; and a literature review, systematic review, and meta-analysis of outcomes in 31,392 patients with chronic wounds including DFUs, VLUs, and PUs, PRP gel therapy was found to improve the proportion of partially and completely healed wounds and cause less infection to occur than standard wound care treatments.19-22 This PRP gel technique received Food and Drug Administration (FDA) clearance in 2007 for use in wounds such as venous leg, pressure, and DFUs and for the management of mechanically or surgically debrided wounds.23 Patients sensitive to any of the PRP gel components or bovine materials should avoid using the product. The PRP gel should not be used on wounds with a malignancy or in patients receiving chemotherapy.
The purpose of this retrospective, longitudinal study was to assess treatment outcomes in Japanese patients with complex and severe ulcerations who were treated in WCCs with standard wound care treatments and PRP gel.
Materials and Methods
A retrospective, longitudinal study design was used to compare treatment outcomes before and after application of the PRP gel in patients with complex and severe ulcerations managed in WCCs. WCCs are partnerships between the hospitals and the Millennia Corporation Inc, Tokyo, Japan. Per policy, data from patients managed by WCCs are collected and stored in a secured database maintained by Millennia Corporation Inc; patient privacy is always protected. For the purposes of analysis, the dataset was de-identified in compliance with the Japanese Private Information Protection Law, which is similar to the US Health Insurance Portability and Accountability Act (HIPAA) regulation.
Before receiving treatment, all patients provide informed consent. Internal review board approval was obtained at each site for general data retrieval, as well as specific to this endeavor. Patient data are entered into a central programmed database and all calculations (eg, wound area) are performed upon data entry.
Study inclusion/exclusion criteria. The entire database was searched for wounds meeting all inclusion/exclusion criteria. Study inclusion criteria required wounds be treated with standard wound care, including the use of dressings and gels that support moist wound healing, use of silver-impregnated or other dressings that inhibit infection, wound bed preparation, addressing underlying factors, and the use of alternative modalities such as negative pressure wound therapy and maggot therapy when appropriate and PRP gel between April and November 2010. Patients who had nonhealing wounds with exposed bone and/or inflammation or infection (ie, greater than or equal to Wagner III for diabetic wounds) at first clinic visit were eligible for inclusion. Lower extremity wounds that were increasing in size and depth and/or did not show signs of healing (ie, absence of granulation tissue deposition); wounds with deep undermining and soft tissue and bone involvement in the foot following extensive debridement or forefoot partial amputation (at presentation and/or past case review); and wounds with ASO were included. Persons with multiple wounds also were eligible. Chronic wounds not treated with PRP gel and wounds that showed signs of healing following standard wound care were excluded. Patient and wound data were retrieved and copied into a separate study database.
Patient and wound data. All hospital WCC staff are trained to use and follow the Millennia Wound Management Program. Standardized assessment forms are used and all data and wound images are entered into a database to track healing outcomes, including patient demographic information and duration and severity of the underlying disease; comorbitities; vascularity data and revascularization procedure history; wound history, size, treatments, and therapies; and diagnostic test results.
Wound care procedures. All patients in the WCC received treatments in an inpatient setting. In addition to the wound specialty team, every hospital has specialty doctors (eg, vascular surgeon, endocrinologist) to participate in patient care. Outpatient care is regularly provided between hospital stays.
The standard treatment algorithm requires all patients to be assessed, including medical history, physical exam, wound assessment, and infection assessment, regardless of wound etiology. In addition, diagnostic tests and noninvasive and invasive vascular studies are performed. Based on the results of the assessments, need for infection control intervention, revascularization, excision, and debridement, growth factor/PRP gel therapy, skin graft/flap, and protection and education is determined. The patient and wound outcomes are reassessed at each visit with frequency determined by the state of the wound. Patients with healed wounds receive additional education about wound severity, the nature of the wound, risks such as amputation, offloading, glycemic control, and the like. Nonhealing wounds are reassessed and interventions reviewed and revised (eg, a wound responding well to treatment might be seen once a week; a nonresponding wound could be seen daily). First-line interventions include infection control typically using systemic antibiotics and/or silver-impregnated dressings, surgical excision and debridement, and/or appropriate dressings, such as hydrogels, absorptive cotton, and synthetic or foam sheets or pads; more advanced interventions involving growth factors and PRP gel therapy are used less frequently. Usually, a chronic wound triggers advanced therapy when it does not respond to standard of care (ie, >30 days old).
PRP gel. PRP gel is made by using approximately 20 mL of the patient’s blood spun for 60 seconds in a specially designed centrifuge calibrated to maximize the PRP. Only the PRP fraction is transferred into a mixing chamber and combined with ascorbic acid and then mixed with calcified thrombin in a standardized ratio to activate the platelets and form a gel containing a fibrin matrix. When the PRP liquid is converted to a clear gelatinous consistency (usually within 15 to 30 seconds), it results in a standard formulation of PRP gel. The wound bed is debrided in the OR under anesthesia and cleansed with normal saline. The gel then is applied topically by the physician in a uniform layer. The wound then is covered with a nonabsorbent contact layer followed by a moisture vapor-permeable film dressing and a secondary absorbent dressing to manage any strikethrough. The PRP gel is not used as cavity filler, only as a thin primary wound contact layer. Depending on the wound characteristics per the WCC algorithm, PRP gel is typically applied once a week until healing occurs. Occasionally, twice-weekly applications are used at the discretion of the clinician. PRP gel therapy might be stopped after several applications if it is determined the wound is not responding.
Wound measurements. Wound measurements are taken and recorded before every dressing change after the dressing has been removed and any debridement performed by the treating clinician, who was previously trained to use a comprehensive wound measurement technique to ensure uniformity. Disposable paper rulers with centimeter markings and cotton-tipped applicators are used to probe and measure length, width, and depth of the visible wound as well as undermining, sinus tracts, and tunneling. Measurements of length, width, and depth are taken consistently at the longest points. Wound area and volume are calculated in the central database using L x W for area and L x W x D for volume.
Infection. Infection is defined as the presence of an abscess, cellulitis, osteomyelitis, and/or gangrene. Infection status was assessed at each visit based on clinical signs and symptoms of inflammation; when needed, additional testing (usually by culture) was performed.27 Wagner DFUs grades III and IV were considered infected. Appropriate empirical antibiotic therapy was started upon diagnosis of infection and revised as necessary.
Vascular interventions. Generally, wounds are classified in WCC program as ischemic based on a variety of clinical symptoms such as problems with lower extremity pulses (eg, femoral bruit), absence of pedal pulse, cool skin, delayed capillary and venous filling, claudication and ischemic rest pain, ankle brachial index (ABI) of <0.9, and/or skin perfusion pressure (SPP) measurements. SPP measurements were taken using a SensiLase PAD3000 (Vasamed, Inc, Eden Prairie, MN) at the dorsal and plantar aspect of the foot around the wound. Three definitions for ischemia were found in the literature and were used clinically depending on clinician preference and training.25,26 Thus, ischemic wound data were analyzed by clinical diagnosis and classified as SPP <40 mm Hg and SPP <30 mm Hg, as well as by overall SPP values for all wounds. Generally speaking, ASO or PAD is clinically diagnosed, but its severity is often assessed using ABI or SPP. SPP, “the new kid on the block” for the WCCs, has not been incorporated yet in all clinical practice guidelines. The cut-off of an SPP <30 mm Hg tends to be more accepted than the higher cut-off point, but larger validation studies are required. Thus, while the diagnosis of ischemia was made primarily using clinical symptoms in this study, researchers also looked at different definitions of ischemia by SPP for purposes of modeling. Revascularization procedures are documented as to the type and number of procedures as well as when they were performed. Procedures were classified as percutaneous transluminal angioplasty (PTA) or an arterial bypass.
Wound status and classification. In the WCC protocol for this study, chronic (nonhealing) wounds were defined as wounds that have been present for >4 weeks or failed to heal through an orderly sequence of events.24 These wounds did not respond to standard wound treatment or were progressively deteriorating.
Healing. Healing wounds were defined as wounds that decrease in size and depth and/or showed signs of healing (eg, deposition of granulation tissue) and a healed wound was defined as complete closure. All wounds are classified by their primary etiology, but some wounds exhibit more than one type such as ischemic diabetic ulcers. Wound classes used for this study were diabetic, ischemic, ischemic diabetic, and pressure ulcer.
Data analyses. All statistical analysis was performed using Predictive Analytics Software (PASW) 19 (SPSS, Inc., Chicago, IL, USA) with an alpha <.05 regarded as statistically significant.
To compare median times to healing, wounds were divided into run-in and PRP gel treatment blocks with time to event (healing) calculated as date of healing — date of first run-in or treatment visit for each block and the log rank test were applied using Kaplan-Meier (KM) time-to-event analysis. Three assessment time points were developed for wound variables. T1 was the beginning of the run-in period when standardized wound care was provided. T2 was the time at first treatment with PRP gel. T3 was the time point at which the wound healed or treatment was discontinued. Percent change in area, depth, or volume between T1 and T2 and T2 and T3 were analyzed using paired Wilcoxon signed rank tests (eg, DPA12 = 100 – ((AT2 /AT1)*100), where DPA12 represents percent area change between T1 and T2, and DPA23 = ((AT2 /AT1)*100) – ((AT3 )/AT1)*100), where DPA23 represents percent area change between T2 and T3, and AT1 represents the area at T1, AT2 the area at T2, and AT3 the area at T3. Because the Millennia clinical database is a compilation of clinical practice, assessment times were not the same for different wounds (ie, for one wound, T1 might be 21 days, while for another it might be 36 days).
To determine which factors influenced time to healing, a Cox regression was performed. Patient age had a non-normal distribution, so it was transformed into an ordinal two-level factor using 70 years of age as the break point. Number of wounds (how many other wounds each person had) had too few values to qualify as a covariate, so it was transformed into a two-level factor (one wound or more than one wound). For the purposes of using a proxy variable based on Wagner grade, an ordinal variable termed infection level was used in which 1 = moderate infection and 2 = severe infection, equating to Wagner III/IV. (Authors’ note: Wagner grading would not normally be used to describe non-DFUs.) In this case, it is used as a way to describe level of exposure and infection, not necessarily etiology.

A two-level nominal factor called revascularization was created, which was scored as no PTA or bypass or PTA/bypass done after the end of the study and PTA/bypass before or during PRP gel treatment. Number of PRP gel treatments also had a non-normal distribution (tested using the Wilk-Shapiro test), so it was transformed into an ordinal three-level factor with the following values: level 1: one to three treatments; level 2: four to eight treatments; level 3: nine or more treatments. Wound duration before PRP gel treatment (the sum of the run-in duration and age of the wound before the first visit at run-in) had a non-normal distribution, so it was log transformed into a normal distribution. T1 area (area at first run-in visit) had a non-normal distribution, as did T2 area (area at first PRP gel treatment; baseline); however, whereas log T1 area still had a non-normal albeit better distribution, log T2 area was satisfactory and was used in the regression. Because T2 area was selected as an initial covariate in the regression, T2 depth had to be selected. This variable, too, had non-normal distribution but was successfully transformed using logs.

As a variable in regression, one of four options could be selected to represent the presence of ischemia: 1) clinical diagnosis in which intermittent claudication and ischemic rest pain are the major symptoms (a dichotomous Yes/No variable; 2) the SPP value itself as a continuous variable; or 3) the SPP in which a cut off point or diagnostic threshold is used to determine whether a wound is ischemic (also a dichotomous variable).28 Two well-known diagnostic studies25,26 (level of evidence III-1 and III-2) have used 30 mm Hg and 40 mm Hg as cut-off points. One cannot use all four possible variables in a Cox regression simultaneously, because they are likely to be highly correlated, and this would lead to what is called variance inflation, the results of which would be an unstable model and unreliable odds ratios (ORs). So, one variable unique to each analysis was used in each of four model variants in which following variables were entered into each initial model: covariates: log T2 area, log T2 depth, log age of wound prior to PRP gel treatment; factors: subject age, gender, DM (Yes/No), smoking (Yes/No), revascularization, stroke (Yes/No), chronic renal failure (Yes/No), hypertension, other comorbidity, number of wounds, infection level, number of PRP gel treatments, NPWT (prior treatment, Yes/No). Least significant variables were removed one by one until a stable model resulted and all remaining covariates or factors were significant. The correlation matrix also was checked frequently to determine if specific factors or covariates were highly correlated with other covariates/factors. Cox proportional hazards were checked as follows in the final model: time dependence by using log minus log plots, time by variable interactions, and plots of Schoenfeld scaled residuals versus time. An independent t-test was used to compare the predictive healing rate at 4 weeks based on percent reduction in area for wounds that later healed or did not heal.29
Results
Of 1,053 potential participants, data from 39 persons with 40 wounds met the study inclusion criteria. Participants included 30 men (77%) and nine women (23%) with a mean age of 66.8 years (SD:10.60), ranging from 34 to 86 years (see Table 1). Persons with DM comprised 85% (n = 33) of the study population and had been diagnosed with the disease for a mean of 23.9 years (n = 17; SD: 14.43), ranging from 1 to 47 years. Seventeen patients (44%) had a history of chronic renal failure, eight (21%) had a stroke, six (15%) had hypertension, and four (10%) had other comorbidities. With regard to ASO, 16 (41%) had undergone PTA, eight (21%) had an arterial bypass, and five (13%) had both procedures. Four individuals had multiple PTAs, and one had multiple bypasses on different dates because of artery stenosis or patency issues. The mean body mass index (BMI) was 25.9 (SD: 3.75) and ranged between 15.8 and 28.9 (n = 28), showing 50% were a normal weight and 25% each were underweight and overweight.
Of the 40 wounds, 34 (85%) were classified as a complication of DM and 29 (73%) were accompanied by moderate to severe ASO as determined by SPP. Five (13%) were arterial ulcers, and one was a pressure ulcer. Twenty-four (24) (60%) wounds were in patients with both DM and ASO. Only 22 patients presented with one wound. The other 17 subjects had at least one additional wound, but those wounds were not treated with PRP gel and were excluded from the study. One subject had two treated wounds, both of the same etiology.
Of the 34 wounds (foot ulcers) in patients with DM, 26 (77%) were Wagner Grade III and eight (23%) were Wagner IV. All study wounds demonstrated signs of infection at baseline and required appropriate systemic antibiotic therapy. Most participants required extensive surgical procedures to alleviate abscess, osteomyelitis, joint sepsis, or gangrene. Because ischemia was defined by clinical diagnosis (SPP <40 mm Hg, SPP <30 mm Hg, or a covariate — as noted previously, three methods can be used to diagnose ischemia: clinical; SPP < 30 mm Hg, or SPP < 40 mm Hg) — multiple symptoms of ischemia were noted. During clinical examination, 28 wounds (70%) were diagnosed as ischemic. When these limbs were measured using SPP, 25 (63%) were classified ischemic (SPP at <40 mm Hg), and of those 20 (50%) had an SPP <30 mm Hg. The mean SPP covariate for all 40 wounds was 36.94 (SD: 24.02).
The mean wound duration before coming to the WCC clinics was 99.7 days (SD: 107.73; range: 3–365 days; n = 24). The mean wound area at first WCC visit (T1) was 13.4 cm2 (SD: 27.07; median: 4.74; range: 0.01–140 cm2). The mean T1 depth was 0.79 cm (SD: 1.154; median: 0.15; range: 0.1–4.2 cm). The mean T1 volume was 11.5 cm3 (SD: 28.62; median: 1.0; range: .001–160 cm3).
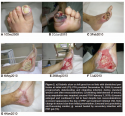
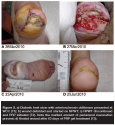
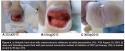
During the run-in period (average 75.3 days, SD 73.35), 14 (35%) study wounds received NPWT and the majority were managed with debridement, offloading, revascularization, infection treatment, and appropriate wound dressings. Figures 2 through 5 show typical examples of wounds seen during the study. At T2 (start of PRP gel treatment), the mean wound duration was 135.1 days (SD: 121.21). The mean T2 area at the first PRP gel treatment was 16.8 cm2 (SD: 26.67; median: 5.16; range: 0.09–114 cm2 ). The mean T2 depth was 1.05 cm (SD: 1.219; median: 0.5; range: 0.1–6.1 cm). The mean T2 volume was 28.2 cm3 (SD: 63.68): median: 2.9; range: 0.01–281 cm3). During an average run-in wound treatment time of 75.3 days, the mean percent increased between T1 and T2 for depth, area, and volume 353%, 881%, and 16,331%, respectively; P values: 0.001, 0.067, and 0.076. The changes based on mean percent reductions are unusually large, because a few small wounds increased enormously (ie, mm to cm) during the run-in period (see Figure 1).
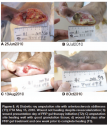
The mean treatment time with PRP gel was 45.4 days (SD: 39.45). Thirty-three of the 40 wounds (83%) healed completely in an average of 145.2 days (SD: 21.29), which was statistically significant (P = .00002). Mean changes over time in area (P = 5.0 x 10-7), depth (P = 1.2 x 10-6), and volume (P = 7.3 x 10-5) were all statistically significant (see Figure 1). The mean number of PRP gel treatments was 6.1 (SD: 3.88; median: 5; range: one to 17). Of the 24 DM/ASO wounds, 19 (79%) healed during an average of 108.1 days (SD: 107.2). Figures 2 through 5 show typical treatment outcomes seen during the study.
Of the seven individuals who did not heal, two died (due to non study-related comorbidities), two terminated treatment, two transferred out of treatment/clinic, and one underwent amputation.
 Survival distributions (time to healing) between the run-in and treatment time periods were significantly different (Mantel-Cox Log rank, P = 0.00002) (see Figure 6).
Survival distributions (time to healing) between the run-in and treatment time periods were significantly different (Mantel-Cox Log rank, P = 0.00002) (see Figure 6).
Cox regression analysis of time to healing showed that smoking (Yes or No) and other comorbidities were highly correlated values with other variables within the correlation matrix. Inclusion of these variables also led to unstable beta coefficients with very high ORs. Their removal tended to stabilize any model. Consequently, they were not included in model refinement. Three factors (hypertension, revascularization, and number of PRP gel treatments) had marginally significant time dependence but were not modeled with respect to time as plots showed that these issues were caused by the relatively small sample numbers in one of the levels of the factors. The final regression model had two covariates and four factors with a –2 log likelihood of 136.67, chi square = 47.54, P = 4.4 x 10-8 (see Table 2).
 Use of ORs for variables determined the odds of healing to occur. The OR for area at first visit for PRP application (T2) was 0.429, meaning that for every increase in an order of magnitude of area, the odds of healing diminish by 0.429 (see Table 2). Likewise, the OR for age of wound at T2 was 0.106, meaning that for every increase in an order of magnitude of time, the odds of healing decrease by 0.106. Having hypertension diminished the odds of healing by 0.005, but having revascularization increased the odds of healing by 5.02, meaning that a prior revascularization procedure increases the odds of healing. An increase in the number of PRP gel treatments decreased the odds of healing (OR = 0.499 [four to eight treatments] and 0.060 [fewer than eight treatments]); this corroborates the data that showed wounds that received more treatments had an increase in severity and had more comorbidities. The OR for severe infection level/Wagner grade IV compared to an infection level of moderate/Wagner grade III was 14.62, which means that the odds of healing were increased by 14.62 when moderate infection level/Wagner grade III wounds were used a reference.
Use of ORs for variables determined the odds of healing to occur. The OR for area at first visit for PRP application (T2) was 0.429, meaning that for every increase in an order of magnitude of area, the odds of healing diminish by 0.429 (see Table 2). Likewise, the OR for age of wound at T2 was 0.106, meaning that for every increase in an order of magnitude of time, the odds of healing decrease by 0.106. Having hypertension diminished the odds of healing by 0.005, but having revascularization increased the odds of healing by 5.02, meaning that a prior revascularization procedure increases the odds of healing. An increase in the number of PRP gel treatments decreased the odds of healing (OR = 0.499 [four to eight treatments] and 0.060 [fewer than eight treatments]); this corroborates the data that showed wounds that received more treatments had an increase in severity and had more comorbidities. The OR for severe infection level/Wagner grade IV compared to an infection level of moderate/Wagner grade III was 14.62, which means that the odds of healing were increased by 14.62 when moderate infection level/Wagner grade III wounds were used a reference.
An independent t-test was performed on the percent change in wound area at 4 weeks for healed and unhealed wounds to determine the predictive value for complete healing. The percent change in wound area at 4 weeks for healed wounds was 62.3% (SD: 44.41) and 10.1% (SD: 37.76) for nonhealed wounds (P = 0.006).
Discussion
Observational descriptive studies can offer clinicians insight into how to best provide care for complex patients with multiple comorbidities that may be too rare or too complex to be included in a typical randomized clinical study.30 The longitudinal study design used included abstraction of standard care and PRP gel treatment outcomes in patients with severe, chronic, and complex wounds facilitating a comparison of pre- and post-PRP gel treatment outcomes. This is the first study to follow such severe, complex wounds to complete healing using PRP gel. During the standard care treatment time, average wound area, depth, and volume increased, and none of the 40 wounds healed over a mean period of 75.3 days. The increase in all wound dimensions was indicative that the wound was deteriorating, not improving. KM analysis of the PRP gel treatment period indicated that wounds treated with PRP gel improved significantly from the pretreatment run-in period, with 33 wounds achieving complete closure.
Even though this study population was small, the healing trajectories were similar to those achieved with PRP gel in much larger study populations (n = 468).17,19-21 An RCT19 conducted in 72 DFUs showed that common-size ulcers treated with PRP gel healed significantly more than their control gel counterparts (81.3% versus 42.1%, P = 0.036). In an observational case series20 of 285 DFU, VLU, and PU wounds, an average 63.6% volume reduction and 47.5% area reduction was seen in 2.2 weeks with 2.8 treatments. Similarly, an observational case series21 of 46 wounds on 34 patients with run-in data without progress resulted in average 33% area reduction and 44% depth reduction in an average of 3.2 weeks. Lastly, a multicenter case series17 of 65 wounds resulted in average 62% volume reduction and 50.9% area reduction in 2.8 weeks with 3.2 treatments. The results of this study also showed that smaller wounds of shorter duration correlated with faster healing than larger, older wounds. Less severe wounds of patients who had successful revascularization procedures also healed more expediently than severe wounds in patients with successful revascularization in retrospective13 (n = 282) and prospective31 (n = 480) observational CLI studies.
The observation that the time to healing OR was higher in patients with a higher number of PRP gel applications is not surprising, because more applications were used in wounds that took longer to heal. However, the high OR for time to healing wounds with a severe infection compared to those with a moderate infection is unexpected. It is possible that these patients received a higher level of care for their infected ulcer, an example of a halo effect.32 It is also possible that because wound infection was coded as present or absent rather than by severity, risk associated with severe infections vs. moderate infections could have been different than the OR of presence of infection. All wound etiologies were assessed using the Wagner scale as a determinant of both wound severity and infection severity rather than etiology. As a result, even though the etiologies were not homogenous, the scale provided a consistent tool for evaluating the extent of the damage and infection.
Long-term studies of patients with severe ischemic wounds are rare, making comparisons difficult. However, some studies provide insight on healing expectations and LEA rates for these types of wounds. A prospective cohort study33 of 2,511 Wagner I through V DFUs treated with standard care that included surgical debridement, appropriate antibiotic systemic therapy, offloading, compression, wound dressings (ie, foam, hydrogels, hydrofibers, hyaluronic acid, silicone, or hydrophobic gauze) and/or topical antimicrobial agents (ie, silver or cadexomere iodine), followed patients until healing or death. Although the overall percentage of wounds healed was high (90.6%, median time to healing 15 weeks), healing rates without amputation were low in patients with Wagner III (n = 251, 7% healed) and Wagner IV and V DFUs (n = 151, 2% healed). More than 50% of patients with these severe ulcers underwent amputation. In the current study, the amputation rate was 2.5% (one patient). A retrospective review34 of 98 Wagner I through IV ulcers with CLI found that following endoluminal angioplasty and minor amputation, 89% of Wagner I and II ulcers and 67% of Wagner III and IV ulcers were healed at 3 months’ follow-up. Four of the Wagner III and IV group required major amputation, even though 84% of the limbs in this group had patent arterial inflow; after 3 years’ follow-up, the presence of Wagner III and IV ulcers was negatively associated with successful limb salvage.
A retrospective study35 of 334 CLI cases (Fontaine III, related to ischemic rest pain, and IV, which presents with ulceration or gangrene) following infrainguinal bypass were evaluated for wound healing at 6 and 12 months. Healing for all wounds was 42% at 6 months and 75% at 1 year. The median time to healing was 173 days. The only significant factor to predict poor wound healing was lesion severity at baseline. A prospective observational study31 (N = 480) with CLI (Rutherford IV, which has ischemic rest pain, V presents with minor tissue loss, and VI, ulceration and gangrene) evaluated healing and LEAs following revascularization. After 30 days, the amputation rate was 2%, mortality 0.8%, and healing 8%, whereas at 1 year, amputation was 50.4%, mortality was 50%, and healing was 14.5%. Factors associated with limb salvage failure were Rutherford V or VI, lesions >2 cm2, infection, gangrene, major amputation, and re-operation.
In the current study, none of the Wagner III (deep ulcer with cellulitis or abscess and osteomyelitis) and IV (localized gangrene) diabetic ulcers and ischemic ulcers healed during the 75.3 day run-in period of standard care treatment, which included appropriate revascularization. Following PRP gel therapy, 83% had complete healing within 145.2 mean days (median 105 days). After a median of 98 days, 79% of wounds with DM and ASO were healed. In this study, only one patient required amputation (2.5%). Compared to the outcomes cited in the literature, these results are encouraging.
Previous studies have looked at DFU percent change in area at 4 weeks from baseline as a predictor for complete healing at 12 or 20 weeks.29,36 A prospective study29 of 203 Wagner I and II DFUs with an average baseline area of 2.8 cm2 found the midpoint of 53% percent change in area at 4 weeks between healed (82%, mean 1.5 cm3) and nonhealed (25%, mean 0.8 cm2) wounds was a robust predictor of healing by week 12. Contrarily, a retrospective study36 of 120 DFUs with an average baseline area of approximately 1.28 cm2 found 50% change in area at 4 weeks was a less robust predicator than 90% at week 8. In the current comparator study of Wagner III and IV DFUs with an average baseline area of 16.8 cm2, the mean percent change in wound area at 4 weeks between healed (62.3%) and nonhealed (10.1%) wounds was 52.2%, similar to the median point found in the prospective study mentioned. In addition, wound severity and size in this study were much greater than most prospective trials, yet at week 4, the percent change in area was 62.3% for healed wounds and only 10.1% for nonhealed wounds. Complete wound closure occurred at an average of 17 weeks. Compared to the published Wagner I and II study at week 4, the percent change in area for healed (82% versus 62.3%) and nonhealed (25% versus 10.1%) Wagner III and IV wounds were statistically more sensitive. As expected, the absolute change in area was greater in this study for healed (1.5 cm3 versus 10.47 cm3) and nonhealed (0.8 cm3 versus 1.7 cm3) DFUs.29 The median absolute change in area at 4 weeks was slightly greater in the prospective than the retrospective study for healed (1.5 cm3 versus 1.33 cm3) wounds but similar in the nonhealed (0.8 cm3 versus 0.86 cm3) DFUs.29,36 It is possible the predicator 50% change in area at 4 weeks is more sensitive for wounds larger than 2 cm2, because small wounds do not require large changes in absolute size to effect a large change in area percent.
Limitations
This was a retrospective study with a small sample size and a relatively large number of variables. In addition to the limitations inherent in retrospective studies, the ORs in the Cox regression model are likely to be inflated (ie, exaggerated). Moreover, some small violations of three variables occurred with respect to hazard proportions over time. The patients were quite different in many respects compared to typical US wound care populations, as evidenced by the BMI distribution, for example,37 and thus it may seem strange that hypertension interfered with wound healing, yet chronic renal failure had no effect. This could be due to the nature of having multiple severe comorbidities in which one dominates over another or the presence of other unknown confounding variables, which affected the modeling. Thus, while the authors believe the specific predictions are likely to be valid in this particular population in a qualitative sense, they may not be quantitatively valid in other populations. Similarly, the overall study design limits the ability to generalize the outcomes observed beyond this population, but adds to the existing evidence related to the effects of this PRP gel on wound healing. Lastly, the variability in the time between the T1, T2, and T3 endpoints, as well as the widely ranging age of these chronic wounds at T1, could be seen as a limitation if treatment times were being compared between study subjects. However, in this study, clinical outcomes for the run-in and treatment periods are compared within each subject. Hence, the variability in times is not an issue.
Conclusion
The results of this retrospective, longitudinal study in patients with long-standing chronic wounds and a history of DM and ischemic disease suggest that good healing outcomes and a low amputation rate can be obtained with a protocol of supportive care (including revascularization procedures) and the PRP gel treatment. Whereas most wounds increased in size during an average of 75.3 days receiving standard care, 83% of these wounds healed an average of 145 days following the addition of this PRP gel to their treatment regimen. Only one patient required an amputation. OR for time to healing showed that larger wounds and wounds of longer duration took longer to heal than smaller wounds of shorter duration. Hypertension also increased the risk of longer time to healing, but having a revascularization procedure reduced the OR healing time. The results of this study provide important clinical information about the outcomes of complex wounds and suggest that the current WCC treatment protocols may help reduce the rate of LEAs in Japan. Prospective controlled studies comparing the use of this PRP gel to other advanced treatments are warranted.
Acknowledgment
Funding for manuscript preparation was provided by Cytomedix, Inc (Gaithersburg, MD). Data were analyzed by Marissa J. Carter, PhD, MBA, Strategic Solutions (Cody, WY); and the manuscript was drafted by Laura K. S. Parnell, Precision Consulting (Missouri City, TX).
Dr. Sakata is Chief of Surgery and Medical Director of Wound Care Center, Hokkaido Cardiovascular Hospital, Sapporo-shi, Hokkaido, Japan. Dr. S. Sasaki is Chief Executive Director of Vascular Surgery and Medical Director of Wound Care Center; and Dr. Handa is Medical Director of Surgery, Sendai Social Insurance Hospital, Sendai-shi, Miyagi-ken, Japan. Dr. Uchino is Executive Vice President of Hospital Operations, Chief of Vascular Surgery, and Medical Director of Wound Care Center; and Dr. T. Sasaki is Medical Doctor of Vascular Surgery and Medical Doctor of Wound Care Center, Tokatsu Clinic Hospital, Matsudo-shi, Chiba-ken, Japan. Dr. Higashita is Chief of Cardiovascular Surgery and Medical Director of Wound Care Center, Yokohama General Hospital, Yokohama-shi, Kanagawa-ken, Japan. Dr. Tsuno is Medical Doctor of Gastrointestinal/General Surgery and Medical Director of Wound Care Center, Tonan Hospital, Kochi-shi, Kochi-ken, Japan. Dr. Hiyoshi is Chief of Endocrinology and Metabolism and Medical Director of Wound Care Center, Dr. Imakado is Chief of Dermatology, and Dr. Morimoto is Chief of Bone and Joint Orthopaedic Surgery, Japanese Red Cross Medical Center, Shibuya-Ku, Tokyo, Japan. Dr. Rinoie is Chief of Podiatric Surgery, Methodist Hospital of Southern California, Arcadia, CA; and Medical Director, Millennia Wound Management, Inc, Los Angeles, CA. Dr. Saito is Staff of Podiatric Surgery, Methodist Hospital of Southern California, Arcadia, CA. Please address correspondence to: Chugo Rinoie, DPM, 301 W. Huntington Drive, #300, Arcadia, CA 91007; email: savefeet@verizon.net.
1. International Diabetes Federation. The Global Burden. IDF Diabetes Atlas Fifth Edition. Available at: www.idf.org/diabetesatlas/5e/the-global-burden. Accessed January 19, 2012.
2. Diabetes Net. Diabetic Population in Japan Increase to be the 6th in the World. Office of Diabetes Net. Available at: www.dm-net.co.jp/calendar/2012/016536.php. Accessed Jan 6, 2012.
3. Trans-national Institute of Health and Nutrition. Outline for the Results of the National Health and Nutrition Survey Japan, 2007; Dec. 2008. Ministry of Health, Labour and Welfare. Available at: www.nih.go.jp/eiken/english/research/pdf/nhns2007.pdf. Accessed January 27. 2012.
4. Centers for Disease Control Diabetes data and trends. Available at: www.cdc.gov/diabetes/statistics/complications_national.htm Accessed January 23, 2012.
5. Kumada Y, Ooura T. February 10 as “Foot Care Day”. Office of Diabetes Net. Available at: www.dm-net.co.jp/calendar/2012/016573.php. Accessed January 16, 2012.
6. Wu SC, Driver VR, Wrobel JS, Armstrong DG. Foot ulcers in the diabetic patient, prevention and treatment. Vasc Health Risk Manage. 2007;3(1):65–76.
7. Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, et al. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45(5 suppl):S1–66.
8. Yan BP, Moran D, Hynes BG, Kiernan TJ, Yu CM. Advances in endovascular treatment of critical limb ischemia. Circ J. 2011;75:756–765.
9. Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, et al. Prevalence of lower-extremity disease in the US adult population 40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care. 2004;27(7):1591–1597.
10. Van Belle E, Nikol S, Norgren L, Baumgartner I, Driver V, Hiatt WR, Belch J. Insights on the role of diabetes and geographic variation in patients with critical limb ischaemia. Eur J Vasc Endovasc Surg. 2011;42(3):365–373.
11. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA, 2005;293:217–228.
12. Dang CN, Boulton AJ. Changing perspectives in diabetic foot ulcer management. Int J Low Extrem Wounds. 2003;2:4–12.
13. Sigala F, Kontis E, Hepp W, Filis K, Melissas J, Mirilas P. Long-term outcomes following 282 consecutive cases of infrapopliteal PTA and association of risk factors with primary patency and limb salvage. Vasc Endovasc Surg. 2012;46:123-130.
14. Furukawa M, Shibuya H, Sato S, Tachikawa Y, Sako H. Treatment of CLI: Oita Oka’s Challenge to Limb Salvage by Team Medicine. J Jap Soc Limb Salvage Podiatr Med. 2011;3:43–46.
15. Suissa D, Danino A, Nikolis A. Negative-pressure therapy versus standard wound care: a meta-analysis of randomized trials. Plast Reconstr Surg. 2011;128(5):498e–503e.
16. Reese RJ. Autologous platelet rich plasma (PRP): what do we know? Important concepts relevant to hair restoration surgery. Hair Transplant Forum International. 2010;Jan/Feb:14-17.
17. Frykberg RG, Driver VR, Carman D, Lucero B, Borris-Hale C, Fylling CP, Rappl LM, Clausen PA. Chronic wounds treated with a physiologically relevant concentration of platelet-rich plasma gel: a prospective case series. Ostomy Wound Manage. 2010;56(6):36–44.
18. Jacobson M, Fufa D, Abreu EL, Kevy S, Murray MM. Platelets, but not erythrocytes, significantly affect cytokine release and scaffold contraction in a provisional scaffold model. Wound Repair Regen. 2008;16(3):370–378.
19. Driver VR, Hanft J, Fylling CP, Beriou JM, AutoloGel Diabetic Foot Ulcer Study Group. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2006;52(6):68–74.
20. de Leon JM, Driver VR, Fylling CP, Carter MJ, Anderson C, Wilson J, et al. The clinical relevance of treating chronic wounds with an enhanced near-physiological concentration of platelet-rich plasma gel. Adv Skin Wound Care. 2011;24(8):357–368.
21. Carter MJ, Fylling CP, Li WW, de Leon J, Driver VR, Serena TE, Wilson J. Analysis of run-in and treatment data in a wound outcomes registry: clinical impact of topical platelet-rich plasma gel on healing trajectory. Int Wound J. 2011;8(6):638–650.
22. Carter MJ, Fylling CP, Parnell LKS. Use of platelet rich plasma gel on wound healing: a systematic review and meta-analysis. Eplasty. 2011;11:e38.
23. FDA 510(k) clearance, BK060007. AutoloGel System. Rockville, MD: Cytomedix, Inc; September 20, 2007.
24. Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Pecoraro RE, Rodeheaver G, Robson MC. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130(4):489–493.
25. Castronuovo JJ Jr, Adera HM, Smiell JM, Price RM. Skin perfusion pressure measurement is valuable in the diagnosis of critical limb ischemia. J Vasc Surg. 1997;26(4):629–637.
26. Yamada T, Ohta T, Ishibashi H, Sugimoto I, Iwata H, Takahashi M, Kawanishi J. Clinical reliability and utility of skin perfusion pressure measurement in ischemic limbs--comparison with other noninvasive diagnostic methods. J Vasc Surg. 2008;47(2):318–23.
27. Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004;39(7):885–910.
28. Esato K. [Clinical diagnosis of arteriosclerosis obliterans]. Nihon Geka Gakkai Zasshi. 1996;97(7):498–503.
29. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26:1879–1882.
30. Carter MJ, Fife CE, Walker D, Thomson B. Estimating the applicability of wound care randomized controlled trials to general wound-care populations by estimating the percentage of individuals excluded from a typical wound-care population in such trials. Adv Skin Wound Care. 2009;22(7):316–324.
31. Chisci E, Perulli A, Iacoponi F, Setacci F, de Donato G, Palasciano G, Cappelli A, Setacci C. Benefit of revascularization to critical limb ischaemia patients evaluated by a patient-oriented scoring system. Eur J Vasc Endovasc Surg. 2012; Feb 17 [Epub ahead of print].
32. Utter GH, Maier RV, Rivara FP, Nathens AB. Outcomes after ruptured abdominal aortic aneurysms: the “halo effect” of trauma center designation. J Am Coll Surg. 2006;203(4):498–505.
33. Gershater MS, Londahl M, Nyberg P, Larsson J, Thorne J, Eneroth M, Apelqvist J. Complexity of factors related to outcome of neuropathic and neuroischaemic/ischaemic diabetic foot ulcers: a cohort study. Diabetologia. 2009;52(3):398–407.
34. Alexandrescu VA, Hubermont G, Philips Y, Guillaumie B, Ngongang C, Vandenbossche P, Azdad K, Ledent G, Horion J. Selective primary angioplasty following an angiosome model of reperfusion in the treatment of Wagner 1–4 diabetic foot lesions: practice in a multidisciplinary diabetic limb service. J Endovasc Ther. 2008;15(5):580–593.
35. Chung J, Bartelson BB, Hiatt WR, Peyton BD, McLafferty RB, Hopley CW, Salter KD, Nehler MR. Wound healing and functional outcomes after infrainguinal bypass with reversed saphenous vein for critical limb ischemia. J Vasc Surg. 2006;43(6):1183–1190.
36. Warriner RA, Snyder RJ, Cardinal MH. Differentiating diabetic foot ulcers that are unlikely to heal by 12 weeks following achieving 50% percent area reduction at 4 weeks. Int Wound J. 2011;8:632–637.
37. Sen CK,Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human Skin Wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–771.













