Negative Pressure Therapy for Complex Wounds in Patients with Sickle-Cell Disease: A Case Study
Abstract
Sickle-cell disease is the most prevalent genetic disease in the Brazilian population. Lower limb ulcers are the most frequent cutaneous complications, affecting 8% to 10% of the patients. These ulcers are usually deep and may take many years to heal.
Evidence about the effectiveness of systemic or topical treatment of these wounds is limited, apart from stabilization of the anemia. A 28-year old woman with sickle-cell disease was admitted for treatment of three deep chronic lower leg ulcers. All wounds had tendon exposure and contained firmly adherent fibrin slough. Following surgical debridement and before grafting, the wounds were managed with three different dressings: a rayon and normal saline solution dressing, a calcium alginate dressing covered with gauze, and negative pressure therapy. All three wounds healed successfully and their grafts showed complete integration; only the rayon-dressed wound required a second debridement. The alginate and rayon-dressed wounds recurred after 9 months and required additional skin grafts. Helpful research on managing ulcers in patients with sickle-cell disease is minimal, but the results of this case study suggest that topical treatment modalities may affect outcomes. Research to explore the safety and effectiveness of NPT in patients with sickle-cell wounds is warranted.
Potential Conflicts of Interest: none disclosed
Sickle-cell disease is the most prevalent genetic disease in the Brazilian population.1 Lower limb (LL) ulcers are the most frequent cutaneous complications, affecting 8% to 10% of the patients. Often seen in the malleoli, these ulcers are usually deep wounds with elevated borders and hyperpigmentation and may contain large amounts of necrotic tissue. Biopsy findings are nonspecific, showing sickle-cell erythrocytes inside the blood vessels of the dermis.2
The mean time to complete healing of these ulcers has been reported to be more than 3 years, which is three to 16 times longer than for wounds of other causes.3 In addition, some case studies4 report recurrence rates that range from 25% to 97%. As a result, these ulcers are classified as complex wounds and their management is considered a challenge.5
Treatment of such complex wounds remains controversial. Despite the diversity of clinical approaches that have been proposed so far, which include simple topical care as well as high technology dressings,6 recurrence rates are still high during the first year post-treatment.7,8 Several systemic therapies also have been tested, such as zinc replacement,9 use of antibiotics,10 pentoxiphylin,11 and blood transfusions.12 However, in the authors’ clinical experience, even with the systemic stabilization of the anemia, these approaches have not produced substantial changes in the wounds, which continue to present raw surface areas and necrosis.
Trying to determine the cost and outcomes of care in patients with sickle-cell anemia wounds, Cackovic et al13 performed a retrospective study of 18 patients using various modalities over a mean duration of 53.7 months. No consistent results were found in the treatment of sickle-cell leg ulcers, but moist dressings provided the best outcomes.
Some case reports6 propose the use of surgical debridement and skin grafting without any kind of wound bed preparation. Although initial results are positive, recurrence rates were high, although the study does not specify exactly how high. A case series by Weinzweig et al14 reported use of microsurgical flaps in five patients with sickle cell ulcers but some ulcers recurred at an unspecified rate.
Recently, negative pressure therapy (NPT) has been used to prepare the wound bed for skin graft. Horch et al15 reported a case series of 21 multimorbid patients, 46 to 80 years of age, with severe lower limb soft tissue loss and infection with exposed bone. Repeated surgical debridement was followed by vacuum-assisted closure therapy at 125 mm Hg continuous mode and subsequent split-thickness skin grafting procedures. In all 21 patients, the wounds healed without a free-flap transfer. The purpose of this case study is to describe the use of a similar protocol of care — debridement, NPT, and skin grafting — on a patient with a sickle-cell ulcer.
Case Study
Ms. C, 28 years of age, of African descent, with sickle-cell disease diagnosed in 1998 and no other comorbidities, was admitted to the chronic wound outpatient clinic of the Clinics Hospital of the Faculty of Medicine of the University of São Paulo (HCFMUSP) in 2005 with nonhealing wounds of 5 years’ duration. At that time, she was receiving folic acid (hydroxyurea) and deferoxamine mesylate as part of the treatment for her disease.
On March 15, 2007, Ms. C was hospitalized with three deep wounds located on the right medial malleolus (5 cm x 10 cm), left medial malleolus (5 cm x 10 cm), and left lateral malleolus (7 cm x 12 cm). All three wounds had tendon exposure and contained fibrin slough firmly adherent to the wound bed (see Figure 1). Ms. C received erythrocyte concentrates only on admission due to low hemoglobin levels (7.2g/dL). The wounds were debrided surgically on day 2 (March 16, 2007). As part of the wound bed preparation, the left lateral malleolus wound was covered with a rayon and normal saline solution (0.9%) dressing, the left medial malleolus wound received a calcium alginate dressing covered by gauze, and the right medial malleolus wound was treated with NPT (125 mm Hg pressure in continuous mode) (see Figure 2). The idea was to evaluate the potential benefits of three different wound bed preparation approaches. 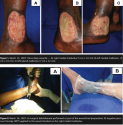 On day 3, the rayon dressing was removed and changed every day thereafter; the alginate dressing also was removed on day 3 and subsequently changed every 48 hours. NPT was applied for 7 days.
On day 3, the rayon dressing was removed and changed every day thereafter; the alginate dressing also was removed on day 3 and subsequently changed every 48 hours. NPT was applied for 7 days.
On March 23, 2007, assessment of all wounds revealed satisfactory granulation tissue — ie, red, beefy tissue with no exudate — on the wound beds that had received alginate and NPT. The wound with the rayon dressing had a considerable amount of fibrin and required a second debridement. Macroscopic analysis showed that, compared to the other wounds, the NPT-treated wound bed had a more homogeneous surface, with better vascularization (see Figure 3).
A split-thickness meshed skin graft (1:1.5) was removed with an electric dermatome from the donor site on the right thigh. The donor site was covered with transparent polyurethane film and the applied skin graft covered with a tie-over dressing. All wounds received skin grafts. Ms. C was transferred to the intensive care unit (ICU) on the second day after surgery due to severe post-transfusion hemolysis, which was treated with transfusion of erythrocyte concentrate, corticosteroids, and heparin. She stayed in the ICU for 5 days for stabilization.
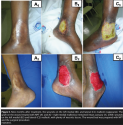
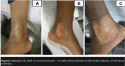
Six days after surgery, the grafted areas were exposed, showing complete integration of all grafts. Ms. C was discharged from hospital after 30 days, to continue follow-up in the outpatient clinic (see Figure 4). 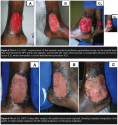 Approximately 9 months after treatment, the wounds on the left lateral and medial malleoli reappeared and were resistant to the usual clinical therapy of saline-soaked gauze. However, the graft on the wound treated with NPT remained intact. On January 16, 2008, the two fibrinous wounds on the left lateral and medial malleolus measured 7 cm x 5 cm. The wound beds were prepared for grafting using NPT (125 mm Hg, continuous mode) and the surgical procedure repeated. On January 24, 2008, the skin grafting was performed (meshed, 1:1.5). The grafts were completely integrated and Ms. C was discharged in 15 days (see Figure 5), without complications or transfusions. She attended the outpatient clinic for follow-up until October 15, 2009, without any recurrent wounds (see Figure 6).
Approximately 9 months after treatment, the wounds on the left lateral and medial malleoli reappeared and were resistant to the usual clinical therapy of saline-soaked gauze. However, the graft on the wound treated with NPT remained intact. On January 16, 2008, the two fibrinous wounds on the left lateral and medial malleolus measured 7 cm x 5 cm. The wound beds were prepared for grafting using NPT (125 mm Hg, continuous mode) and the surgical procedure repeated. On January 24, 2008, the skin grafting was performed (meshed, 1:1.5). The grafts were completely integrated and Ms. C was discharged in 15 days (see Figure 5), without complications or transfusions. She attended the outpatient clinic for follow-up until October 15, 2009, without any recurrent wounds (see Figure 6).
Discussion
It is estimated that more than 2 million people in Brazil have the hemoglobin S gene and more than 8,000 have the severe form of sickle-cell disease (homozigosity). Every year, approximately 700 to 1,000 new cases of sickle-cell disease are diagnosed.16
HCFMUSP has 343 registered sickle-cell disease patients, of which 31 are followed-up in the Complex Wound Outpatient Clinic due to lower limb ulcers. All 31 patients have had their wounds for at least 3 years, and in many cases, the wounds have not responded to topical and/or systemic treatment.
The patient described in this study presented one of the most serious cases followed in the authors’ clinic; the patient had a 5-year history of three deep nonhealing wounds, with necrosis, multiple infections, severe pain, and no response to any kind of therapy. The wounds also were affecting the biomechanics of both lower limbs, impairing ambulation.
Before grafting, a rayon dressing with normal saline, a calcium alginate dressing, and NPT were provided, one approach per wound. All three wounds healed successfully, and their grafts showed complete integration. However, right from the start, a difference was noted in the quality of the granulation tissue formed on the wound bed of the NPT-treated wound. The granulation tissue had an even surface, which resulted in a cleaner, more superficial, and reddish healthy-looking wound bed. The lesion that received normal saline needed a second debridement before grafting.
Other authors have had similar difficulties preparing the wound bed of this kind of lesion; when simple surgical debridement was not enough, different dressings were tried.8 Rayon with normal saline solution may not be able to maintain a favorable environment to stimulate granulation, facilitating the resurgence of necrotic tissue.
The wound that received the calcium alginate did not require a second debridement. A review of the literature17 suggests that calcium alginate provides a moist microenvironment, facilitating cell proliferation and formation of granulation tissue. This could explain why additional debridement was unnecessary.
Although no difference between the three wounds was observed regarding graft take, the wound treated with NPT was the only one that had not recurred at the last follow-up visit, 11 months after surgery. The other two wounds recurred and required surgical intervention. In addition, during the follow-up period, while all wounds were still closed, the skin on the NPT-treated wound appeared more resilient and pliable than the others. This is the first study to date describing NPT used in a patient with sickle-cell anemia.
The question could be raised whether the NPT-treated wound achieved the best results because that wound was less severe than the other two. However, after the recurrence of the ulcers in the other leg, NPT was used to prepare the wound bed for a new graft and no recurrence was observed so far.
The optimal treatment for ulcers in patients with sickle-cell disease remains obscure due to the lack of controlled randomized studies; however, the results of this case study confirm previous reports suggesting that these wounds respond better in the presence of a moist wound environment.13 The encouraging outcome suggests that research to explore the safety and effectiveness of NPT in patients with sickle-cell wounds is warranted.
Conclusion
In a case study of a 28-year-old African women with sickle-cell disease and three complex chronic wounds, using NPT before skin grafting resulted in a healed wound with no recurrence thus far; whereas, wounds on the contralateral leg managed with saline-moistened rayon or a calcium alginate dressing before grafting recurred after 9 months. Studies to increase understanding about the potential role of NPT in the management of wounds in patients with sickle-cell disease are needed.
Dr. Paggiaro is an attending plastic surgeon, Plastic Surgery Division; Dr. Carvalho is a Scientific Research Advisor; Dr. Fonseca is an attending physician in hematology; Dr. Doi works in the Plastic Surgery Division; Dr. Ferreira is Full Professor and Chairman, Hospital das Clinicas, Plastic Surgery Division, Faculdade de Medicina da Universidade de Sao Paulo (FMUSP), Sao Paulo, Brazil. Please address correspondence to: Viviane Fernandes de Carvalho, PhD, ETN, Av.: Dr Arnaldo, 455— Room 1363 — CEP: 01246-903; email: vivianefcarvalho@usp.br.
1. Paladino SF. Úlcera de membros inferiores na anemia falciforme. Rev Bras Hematol Hemoter. 2007;29:288–290.
2. Eckman JR. Leg ulcers in sickle cell disease. Hematol Oncol Clin North Am. 1996;10:1333–1344.
3. Gordon S, Bui A. Human skin equivalent in the treatment of chronic leg ulcers in sickle cell disease patients. J Am Podiatr Med Assoc. 2003;93(3):240–241.
4. Trent JT, Kirsner RS. Leg ulcers in sickle cell disease. Adv Skin Wound Care. 2004;17:410–416.
5. Ferreira MC, Tuma P Jr, Carvalho VF, Kamamoto F. Complex wounds. Clinics. 2006;61:571¬–578.
6. Reindorf CA, Walker-Jones D, Adekile AD, Lawal O, Oluwole SF. Rapid healing of sickle cell leg ulcers treated with collagen dressing. J Natl Med Assoc. 1989;81:866–888.
7. Schleucher R, Gaessler M, Knobloch J. Rapid healing of a late diagnosed sickle cell leg ulcer using a new combination of treatment methods. J Wound Care. 2007;16:197–198.
8. Ballas SK. Sickle cell anaemia: progress in pathogenesis and treatment. Drugs. 2002;62:1143¬–1172.
9. Sergeant GR, Galloway RE, Gueri MC. Oral zinc sulphate in sickle-cell ulcers. Lancet. 1970;2:891–892.
10. Baum KF, MacFarlane DE, Maude GH, Sergeant GR. Topical antibiotics in chronic sickle cell leg ulcers. Trans R Soc Trop Med Hyg. 1987;81:847–849.
11. Frost ML, Treadwell P. Treatment of sickle cell leg ulcers with pentoxifylline. Int J Dermatol. 1990;29:375–376.
12. Fried M, Golan J, Moshe F. Treatment of leg ulcers in sickle cell disease. Blood. 1990:75:2467.
13. Cackovic M, Chung C., Bolton LL, Kerstein MD. Leg ulceration in the sickle cell patients. J Am Coll Surg. 1998;187:307–309.
14. Weinzweig N, Schuler J, Marschall M, Koshy M. Lower limb salvage by microvascular free-tissue transfer in patients with homozygous sickle cell disease. Plast Reconstr Surg. 1995;96:1154–1161.
15. Horch RE, Dragu A, Lang W, et al. Coverage of exposed bones and joints in critically ill patients: lower extremity salvage with topical negative pressure therapy. J Cutan Med Surg. 2008;12:223–229.
16. Silva MC, Shimauti ELT. Effectiveness and toxicity of hydroxyurea in children with sickle cell anemia. Rev Bras Hematol Hemoter. 2006;28:144–148.
17. Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38:563–576.













