Managing Complex, High-Output, Enterocutaneous Fistulas: A Case Study
Abstract
Gastrointestinal (GI) fistulas are an uncommon but serious complication. Following diagnosis, management strategies may have to be adapted frequently to address changes in fistula output, surrounding skin or wound condition, overall patient clinical and nutritional status, mobility level, and body contours.
Following a motor vehicle accident, a 49-year-old man with a body mass index of 36.8 and a history of multiple previous surgeries, including gastric bypass, experienced excessive output from a fistula within a large open abdominal wound measuring 45 cm x 40 cm x 5 cm. Abdominal creases and the need to protect a split-thickness skin graft of the wound surrounding his fistula complicated wound management. During his prolonged 4-month hospital stay, the patient underwent several surgical procedures, repeated wound debridement, and various nutritional support interventions; a wide variety of wound and fistula management systems were utilized. One year after the initial trauma, the fistula was surgically closed. One week later, the patient died from a cardiac event. This case study confirms that GI fistulas increase costs of care and hospital length of stay and require the experience and expertise of a wide array of patient support staff members and clinicians.
Please address correspondence to: Barbara Hahler, MSN, RN, ACNS-BC, CWOCN, Mercy St. Vincent Medical Center, 2213 Cherry Street, Toledo, OH 43608; email: hahlb@yahoo.com.
A fistula is an abnormal passage between two hollow organs or a hollow organ and the skin.1 Fistulas in the gastrointestinal (GI) system are classified by the site of origin and termination, volume of drainage, and etiology. Care of a GI fistula can be very challenging for the healthcare team and initiates a prolonged course of recovery for the patient.2 Fistulas into an open abdominal wound create even more complex management challenges.
GI fistulas may occur after a surgery or spontaneously. An estimated 80% of GI fistulas occur as complications after abdominal surgery3 with an estimated overall incidence of 0.8% to 2%.4 Fistulas-associated morbidity includes malnutrition, electrolyte imbalances, skin excoriation, abscess formation, sepsis, and dehydration.5 The development of enterocutaneous fistulas in trauma patients has been shown to increase length of stay an average of 21 days in the intensive care unit and 66 days in the hospital.6 Direct treatment costs for patients with enterocutaneous fistulas have been found to average $412, 313 or higher.6 Mortality rates are at 5% to 21% for all enterocutaneous tracts and 35% for jejunal fistulas. The predominant causes of death are sepsis, electrolyte imbalance, and malnutrition.7
Factors contributing to the development of postoperative enterocutaneous fistulas are classified as patient-specific or technique-specific.8 Patient-specific risk factors include a history of radiation therapy, inflammatory bowel disease, adhesiolysis, malnutrition, infection, and operations in the emergency setting with possible hypotension, hypothermia, anemia, or poor oxygen delivery.8
 Technique-specific factors related to protocols. According to a literature review8 of enterocutaneous fistula management, the nutritional status of the patient should be assessed preoperatively. Patients with a recent 10% to 15% weight loss and/or an albumin level <3.0 g/dL have been found to be at increased risk for poor healing and possible fistulas development. Anemia can compromise oxygen delivery to the tissues; patients should be normovolemic. Preoperative antibiotic therapy (ie, preoperative bowel and skin preparation) decrease the risk of infection and therefore fistula development.8 During surgery, tension on any bowel anastomosis should be avoided to ensure adequate blood supply. Hemostasis must be achieved, incidental enterotomies avoided, and serosal injuries identified and repaired. An omental flap should be used to shield the anastomosis from the incision. Finally, the abdominal wall should be closed securely to avoid injury to the underlying bowel.8
Technique-specific factors related to protocols. According to a literature review8 of enterocutaneous fistula management, the nutritional status of the patient should be assessed preoperatively. Patients with a recent 10% to 15% weight loss and/or an albumin level <3.0 g/dL have been found to be at increased risk for poor healing and possible fistulas development. Anemia can compromise oxygen delivery to the tissues; patients should be normovolemic. Preoperative antibiotic therapy (ie, preoperative bowel and skin preparation) decrease the risk of infection and therefore fistula development.8 During surgery, tension on any bowel anastomosis should be avoided to ensure adequate blood supply. Hemostasis must be achieved, incidental enterotomies avoided, and serosal injuries identified and repaired. An omental flap should be used to shield the anastomosis from the incision. Finally, the abdominal wall should be closed securely to avoid injury to the underlying bowel.8
Diagnosis. If a fistula develops, its course and nature must be assessed. Methylene blue can be used to confirm the presence of a fistula but may not provide sufficient anatomic information.8
A fistulogram may provide information regarding the fistula source, length, and course as well as the presence or absence of bowel continuity, distal bowel obstruction, or an abscess cavity. Fistulograms are performed by inserting a soft catheter into the fistula and instilling contrast media.8 A CT scan can identify abscesses and may guide percutaneous drainage, but fistulas are not often distinctly seen on CT scans.8 Upper GI studies with small bowel follow-through and barium enemas may be performed but rarely provide more information than the fistulogram.2,8
Fistula output. Fistula output depends on its origin in the bowel — the more proximal the fistula in the GI tract, the larger the volume of output.9 A fistula is considered to be high volume when it has an output >500 mL/day, moderate when volume is 200 mL to 500 mL/day, and low when output is <200 mL/day.9,10 Small intestine fistula output is alkaline and caustic to skin.10
Fistula management. Initial management of a fistula includes diagnosing the fistula and stabilizing the patient. The patient typically has had a poor early postoperative course; many patients experience fever and a prolonged ileus. Usually, wound erythema develops, then purulent drainage, followed by enteric contents that drain from the wound.8
The first goal is restoration of circulation volume. Fistula fluid loss can lead to dehydration; intake and output must be meticulously measured and recorded.8 Fluids must be replaced and patients with high output fistula will need total parenteral nutrition (TPN).2,11 Several recent studies8,12,13 report on the importance of enteral nutrition alone or in combination with TPN. Providing even 20% of caloric need enterally may protect the integrity of not only the mucosal barrier, but also hormone and immunologic functions of the gut. Hepatic protein synthesis also has been found to improve with enteral feeding.8,12
Serum albumin and prealbumin levels facilitate indirect estimate of visceral protein stores that may be affected by the patient’s state of hydration and renal function. The albumin level may take 14 days to return to normal when depleted.14 Prealbumin level reflects acute changes in nutritional status. Although prealbumin levels may decrease in the presence of inflammation and zinc deficiency, as well as in the immediate postoperative period, these levels do not decrease with dehydration and may increase during prednisone therapy.14 Measuring levels of transferrin, the plasma protein for iron transport, also can be helpful in monitoring protein loss.15
In cases of higher, more proximal fistulas, patients are usually not allowed anything by mouth (NPO); proton pump inhibitors are given to reduce stimulation of secretions from the bowel.2,11 A nasogastric tube (NG) commonly is used as a means to reduce fistula output; however, evidence that this intervention is beneficial in the absence of prolonged ileus or obstruction is scant.8
Octreotide is a synthetic analogue of somatostatin, a hormone produced in several areas of the body, including GI mucosa.5 This substance inhibits GI and pancreatic secretions and gall bladder contractility and motility8; it can be administered subcutaneously or intravenously. Evidence from a prospective, controlled, multicentered study16 (N = 40) comparing TPN alone to TPN and octreotide demonstrated a decrease in fistula output and spontaneous closure rates. However, randomized controlled trials17,18 of 31 and 14 patients, respectively, with fistulas that compare the use of placebo (nonoperative therapy) versus octreotide demonstrated that use of octreotide failed to demonstrate a consistent decrease in fistula output or closure rates. An adverse effect is the development of gallstones.19 Gall bladder sludge or asymptomatic gallstones occur in 20% to 50% of patients treated with synthetic analogues of somatostatin.5 Thus, although this hormone may reduce fistula output, simplifying care of these patients, routine use is controversial.8,19 Loperamide and diphenoxylate have been found to be more helpful for distal (colonic) fistulas because they allow better absorption of intestinal contents.10
A review of the literature8 suggests that spontaneous closure occurs in about 30% of patients with enterocutaneous fistulas; 90% to 95% of fistulas that spontaneously resolve do so within the first 4 to 5 weeks. Fistulas in an open granulating wound have a 6% to 10% chance of spontaneous closure.20 Factors that reduce the likelihood of spontaneous closure include high output, ileal location, multiple fistulas, a short fistula tract, a large opening in the bowel, distal obstruction, and the development of sepsis.8,21
While the fistula is closing, containment and quantification of drainage, skin protection, and odor control must be addressed. Drainage can be contained using gauze dressings, pouches, and suction catheters.8 Fistulas with output of 150 mL or less per day can be managed with protective ointments, skin sealants, and skin barriers.22
A high-output fistula often is managed using a pouching system. The location of the fistula — within an incision or in an area of abdominal creases — may complicate pouch application. When a fistula is located within a wound, achieving a secure pouch seal is difficult, requiring innovative dressing techniques to protect and cover the moist granulation tissue around the fistula.21 Suction catheters may be placed in the fistula opening or in the wound base and connected to suction.
Negative pressure wound therapy (NPWT) has been utilized to contain the effluent and promote wound healing when a fistula is located within an open wound.22 Case studies23-25 have shown that NPWT may help reduce small bowel fistula drainage. In one series of three patients treated with NPWT, 66% of fistulas resolved without surgical intervention after an average of 4 weeks.25 Goverman et al26 describe a series of five cases in which NPWT was combined with a pouching system to contain effluent and stabilize split-thickness skin grafts in open abdominal wounds with fistulas.
In all cases, fistulas treatment requires a multidisciplinary approach including medical nutrition services. Often, long hospital stays occur with medical management. Because the open wound and presence of effluent are likely to have a detrimental effect on body image,27 psychosocial support is of great importance. Physical and occupational therapy may be needed to maintain mobility and facilitate activities of daily living.
Surgical closure of a fistula must be a carefully considered option. Major abdominal surgery stimulates the formation of dense adhesions, especially when complicated by intra-abdominal sepsis and fistula formation. Adhesion development is maximal from the third to tenth postoperative week.8 Adequate nutritional status must be ensured before operative management of a fistula. In addition, delaying the operation decreases the risks of multiple enterotomies and difficult dissection that would be present in the immediate postoperative period due to the presence of adhesions.8
Case Study
This case study describes the management of a complex high-output fistula within a huge open abdominal wound in a morbidly obese patient with a history of gastric bypass surgery.
Mr. L was a single, 49-year-old man who had been involved in a motor vehicle accident and admitted to the authors’ tertiary level one trauma center on October 28, 2007. He was wearing a seatbelt at the time of his accident and sustained a severe external seatbelt injury to his abdomen in addition to his internal injuries.
Mr. L’s history included smoking three packs of cigarettes per day, hiatal hernia, gastric bypass procedure, bowel obstruction, midline hernia repair with mesh, right inguinal hernia repair, and shoulder surgery. He also had a history of peripheral vascular insufficiency. At admission, he weighed 130 kg and was 188 cm tall (body mass index 36.8).
Initial CAT scan revealed previous gastric bypass surgery that was suspicious for mesenteric laceration. Following admission, his abdominal area became increasingly tender so he was taken to surgery within 24 hours of the initial injury. The surgery included an exploratory laparotomy with resection of infarcted mid-jejunum (53 cm), end-to-end anastomosis, repair of a mesenteric laceration, lysis of adhesions, and repair of ventral hernia and enterotomy.
Mr. L’s postoperative diagnoses stated infarcted midjejunum, mesenteric laceration, internal degloving of the abdominal wall (fat separated from the fascia in the area underlying his seatbelt), adhesions, and multiple ventral hernias. Pathology revealed ischemic bowel and transmural necrosis. He remained NPO with an NG tube connected to low continuous wall suction at 80 mm Hg. TPN was initiated for nutritional support.
On postop day 6, Mr. L’s bowel sounds decreased, his white blood cell count increased, and increasing cellulitis and erythema were noted around the right side of the wound. The redness increased very rapidly, causing concern he was developing necrotizing fasciitis of the abdominal wall. The surgeon performed bedside wound debridement and continued to closely watch the wound.
Two days later (postop day 8), Mr. L returned to the operating room for resection of the anastomosis (18 cm of small bowel) with irrigation of an abscess cavity, end-to-end anastomosis, and placement of a Baker’s tube. The tube was tunneled through the anastomosis to the abdominal wall and connected to low continuous wall suction set at 80 mm Hg to reduce tension at the site of anastomosis. An area of abdominal skin and soft tissue, approximately 45 cm x 40 cm x 5 cm deep, also was removed due to devitalized fat and a degloving injury. The soft tissue injury was consistent with the location of his seatbelt. Abdominal closure was limited by a previous ventral hernia repair with mesh and the large soft tissue injury. NPWT, using the abdominal pack dressing (Vacuum-Assisted-Closure® [V.A.C.], KCI, San Antonio, TX) was applied with continuous 125 mm Hg pressure at the end of the procedure. The decision was guided by Mr. L’s body habitus and extensive soft tissue damage at the right abdomen, along with the concern that a left-sided ostomy would torque the small bowel and cause anastomosis. An infectious disease consult was ordered at this time relevant to the abscess and continued elevated white blood cell count, which ranged from 16 k/µL to 23 k/µL since admission.
Mr. L returned to surgery 48 hours later for further debridement of the abdominal wall and to change the NPWT dressing. Only a few areas of necrotic subcutaneous tissue and fascia needed to be debrided. At this point, his prealbumin was 1.7 mg/dL and albumin level was 2.1 g/dL.
After 4 days, the surgeon believed Mr. L was stable enough to have the NPWT dressing changed at the bedside. Ischemia of the subcutaneous tissue was noted along with enteric drainage at the wound base. Mr. L was returned to surgery that afternoon for debridement of the abdominal wound, laparotomy, closure of an enteric fistula at the site of the previous anastomosis, closure of the abdominal wall with an acellular dermal matrix (AlloDerm,® Lifecell Corporation, Branchburg, NJ), and application of NPWT, utilizing polyvinyl alcohol (PCA) foam and reticulated foam dressings at 125 mm Hg continuously.
Three days later, the NPWT dressing was changed at the bedside. The wound was 42 cm x 32 cm x 6 cm deep. Recurrence of the fistula was evidenced by enteric-type drainage and gas bubbles in the right side of the wound base. Because the wound was too large for conventional pouches, the NPWT dressing was continued at continuous 125 mm Hg and PVA foam was applied over the acellular wound matrix. Wide-mesh nonadherent gauze and PVA foam were placed over the area of the fistula and three large reticulated foam dressings were used to cover the remainder of the wound. Because the fistula was draining more than 1,500 mL daily, a red rubber catheter also was placed in the wound bed and connected to low continuous wall suction (80 mm Hg) because the NPWT system was unable to contain the large amount of output.
Sixteen days after the last debridement, Mr. L was returned to surgery for debridement of the abdominal wound and to change the Baker’s tube to a jejunostomy feeding tube. During this procedure, areas of loose acellular dermal matrix were removed. The fistula was intubated with a red rubber catheter. NPWT was continued with polyvinyl alcohol foam and reticulated foam dressing (continuous 50 mm Hg). Two NPWT tubes were utilized to collect the large amount of drainage from the fistula. One of the tubes was placed directly over the red rubber catheter and a second tube was placed in the inferior aspect of the wound.
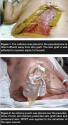
The abdominal wound began to granulate and 5 weeks after the initial trauma, split-thickness skin grafts were applied. Due to the drainage, some loss of the skin graft inferior to the fistula was anticipated, but the approach used was thought to be the best method to obtain final wound closure. A nonadherent antimicrobial gauze was placed over the skin graft followed by the PVA foam dressing for NPWT at 125 mm Hg continuously (see Figure 1). Many variations of NPWT application were tried to manage the high fistula output and prevent loss of the skin graft, particularly on the inferior aspect of the wound. This included use of NPWT in conjunction with an ostomy pouch over the fistula site.
At times, bubbles accumulated in the NPWT canister, setting off the “full” alarm when very little drainage was present and increasing the number of canisters used. Simethicone drops were instilled through the tubing into the canister to reduce the bubbles.
 During this time, TPN was continued for nutritional support. Mr. L’s desire for food was a constant challenge. His social worker, case manager, pastoral care, and the nursing staff provided psychological support. Continuity of care was emphasized and a core of three to four nurses who took a personal interest in his case was routinely assigned. Room decorations, humor, and casual conversations were part of his daily care, which provided distraction and emotional support.
During this time, TPN was continued for nutritional support. Mr. L’s desire for food was a constant challenge. His social worker, case manager, pastoral care, and the nursing staff provided psychological support. Continuity of care was emphasized and a core of three to four nurses who took a personal interest in his case was routinely assigned. Room decorations, humor, and casual conversations were part of his daily care, which provided distraction and emotional support.
After 2 months, the goal became to increase Mr. L’s mobility by removing the catheter attached to wall suction and finding a pouching system to contain the effluent. Experienced WOC nurses tried various pouches, challenged by the changing contours of his abdomen with movement. Fistula output remained high and ranged from 2,500 mL to 3,000 mL per day. Pouch adhesion was further complicated by the presence of moist granulation tissue at the wound base around the fistula. Because of the large amount of effluent, the pouch was attached to suction for the majority of the time but was connected to a straight drainage system when wall suction was disconnected for physical therapy activities. This allowed Mr. L to experience a change in scenery and to go to the gym for physical therapy. Despite trying multiple pouches and modifying the application process, maximum pouch seal duration was usually less than 24 hours. After 10 days, frequent pouch leakage, subsequent skin irritation, and Mr. L’s frustration lead treatment back to NPWT.
A modified T-tube was placed in the fistula stoma by the surgeon and connected to low continuous wall suction at 80 mm Hg to reduce the effluent going onto the wound. Octreotide was tried, but little change in the volume of output was noted; therefore, it was discontinued after several weeks.
Throughout this time, the skin graft was adherent superior to the fistula, but had not taken on the inferior aspect due to constant exposure to fistula effluent. Pectin-based skin barrier (Premium Skin Barrier®, Hollister Corporation, Libertyville, IL) was applied over the mature skin graft to protect it and help epithelial cells migrate inferiorly in the wound.
Almost 3 months after initial trauma, Mr. L’s prealbumin was 7.1 mg/dL. One week later, his NG tube was discontinued for the first time and an elemental tube feeding was started slowly via the jejunostomy tube. Because Mr. L experienced several episodes of line sepsis, removal of invasive lines became another goal of care.
Because many more epithelial cells had migrated over the wound bed by mid January, various pouching systems again were tried. Pectin skin barrier wafers also were continued over the area of maturing skin graft and Mr. L’s clinicians were able to obtain a pouch seal for 24 hours when it was connected to straight drainage.
A long-term acute care hospital or extended care facility was sought for the next stage of his recovery. Caregivers from several institutions came to observe the pouch application and wound care procedures; most were overwhelmed by the complexity of Mr. L’s wound care. Close coordination with Workman’s Compensation was maintained to ensure coverage of the cost of care at the facility selected.
After a 3-month stay, Mr. L was discharged to a long-term care facility with WOC nurses available. A phone conference, written instructions, and pictures were used to communicate pouch application techniques to the new facility’s staff. Mr. L could explain how to apply the pouch but was not able to do this independently due to the complexity of the system. During the first 48 hours after discharge, he experienced multiple episodes of pouch leakage, leading to increased skin excoriation and loss of areas of skin graft; consequently, he was readmitted to the authors’ facility.
Re-admission. Mr. L’s weight at readmission was 116 kg and remained steady during the remainder of his stay. After his readmission, pectin-based skin barriers were continued over the skin graft and NPWT was resumed. However, the effluent was thicker and plugged the NPWT tubing.
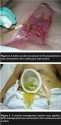
Pouch application was tried again. The major challenge continued to be the large area (12 cm x 18 cm x 1 cm deep) of granulation tissue around the fistula stoma, thwarting product adherence. A pouch, applied over the fistula, was connected to low continuous wall suction at 80 mm Hg and NPWT at 125 mm Hg continuously was applied over the remainder of wound (see Figure 2). This system maintained a seal for 24 hours.
Mr. L was fed a full liquid diet plus tube feeding. His prealbumin was 18.1 mg/dL.
One week following his readmission, the surgeon inserted a button g-tube to stent open the buckled posterior wall of the fistula and allow distal passage of stool. This tube was connected to low continuous wall suction at 80 mm Hg.
The following week, tube feedings were discontinued and a pureed diet was ordered. Mr. L did not find this palatable, and even though pureed, his transit time was extremely rapid; plus, due to his previous gastric bypass surgery, solid food particles would plug drainage tubes. His food then was blenderized several times to eliminate any food particles. This consistency was more like soup and more palatable to him but as a result of his increased oral intake, his fistula output was >4,550 mL/day.
Despite the high fistula output, Mr. L’s prealbumin level stayed within the low normal range, he gained weight, and re-epithelialization of the wound was observed. After 14 weeks, the wound measured 7.5 cm x 9.5 cm x 0.05 cm and the fistula pseudostoma measured 2.5 cm x 2.75 cm, decreasing to 6 cm x 7 cm after 15.5 weeks (see Figure 3). Again, alternate placement for Mr.L was sought. He needed a facility with wall suction (the amount of effluent had increased to >5,000 mL/day and dressings could only be maintained by using wall suction) but few long-term care facilities with wall suction were available in the surrounding area.
 At one point, it was noted the button g tube was no longer in the fistula stoma. Repeated x-rays revealed passage of the modified gastrostomy tube into the large bowel on day 3; it was passed via the rectum.
At one point, it was noted the button g tube was no longer in the fistula stoma. Repeated x-rays revealed passage of the modified gastrostomy tube into the large bowel on day 3; it was passed via the rectum.
By this time, the wound had re-epithelialized enough to pouch outside of the entire wound. The Coloplast Maxi Fistula and Wound Management System™ (Coloplast Corporation, Minneapolis, MN), was applied. This system has a cutting surface of 8 inches x 11.5 inches, larger than most other fistula pouches. It also had a barrier that was extremely flexible and would fit the highly irregular contours of Mr. L’s wound. The pouch has two ports that were attached to low continuous wall suction of 80 mm Hg to contain the large amount of output. Ostomy powder and pectin skin barriers were used over the moist granulation tissue around the fistula stoma and changed daily through the lid of the pouch. The pouch was applied to the intact skin and skin graft and changed every 5 days (see Figure 4). Staff continued to strive to protect the epithelial cells from the effluent with the long-term goal of using a standard ostomy pouch connected to straight drainage so Mr. L could return to his apartment.
Previous attempts to teach self-care had not been successful due to the complexity of the pouching application and the large amount of effluent. Once an adequate seal was obtained with the Coloplast pouch, staff attempted to teach self-care. Because of his obese abdomen, Mr. L could not see the inferior aspect of the wound. He was attentive during the pouch change procedure and he could verbalize the proper steps and needed supplies. Unfortunately, no family members were willing to assist him with his complex care.
Four months after the initial admission, Mr. L was discharged to a long-term care setting that employed a nurse practitioner interested in wound care. Discharge instructions were sent in writing and pictures were enclosed. Mr. L was also able to assemble some equipment and verbalize the pouch application procedure. He was encouraged to be active in the care of the wound. He remained at that facility for 4 months and then was able to be transferred to a long-term care setting closer to his family. He returned to the Trauma Clinic every month for follow-up.
Seven months after the injury, Mr. L’s wound was almost completely re-epithelialized (see Figure 5). Fistula closure was scheduled for 1 year after the trauma because the fistula was close to the ileocecal valve. Absorption of nutrients is markedly improved by retention of the ileocecal valve, and important consideration per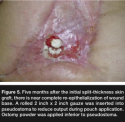 author experience given his history of gastric bypass. Also after 1 year, adhesions should be softer and more easily resected, making incidental enterotomies less of a risk.
author experience given his history of gastric bypass. Also after 1 year, adhesions should be softer and more easily resected, making incidental enterotomies less of a risk.
Fistula Closure
One year after the initial trauma, Mr. L was admitted for fistula closure. His weight was 153 kg, his prealbumin was 20.9 mg/dL, and albumin was 3.8 g/dL. The skin graft tissue was excised in the operating room. The fistula was found to be near the ileal cecal valve, as expected; the right colon was very dilated and avascular. A right hemicolectomy and distal ileum resection were performed and an ileal-to-transverse colon anastomosis was created. An acellular dermal matrix was inserted to close the abdominal wall defect and mobilization of the skin facilitated primary closure.
One week post fistula closure, Mr. L was experiencing an uneventful recovery. His bowel function had returned and he was advanced to a regular diet. Without warning, he developed bradycardia and became unresponsive. All cardiopulmonary resuscitation efforts were unsuccessful.
Conclusion
Treating patients with enterocutaneous fistulas can be a major challenge for patients and their healthcare teams. The reported average length-of-stay of 66 days was exceeded by this patient as a result of extremely high output, thought to have increased as a result of his previous gastric bypass surgery, fistula location, and management challenges that prevented timely transfer to a nonacute care facility. Despite outputs of up to 5,000 mL/day, the patient gained weight and his proteins markers were within the low- normal range. He also did not experience any episodes of dehydration or altered renal function.
His care was repeatedly changed to accommodate this large, complex wound during his 4 months in the acute care setting. A multidisciplinary approach to fistula management was found to be essential. Determining how to best approach the large wound, multiple wound contours, high output, and the patient’s emotional needs involved a complement of skills from a variety of healthcare providers: nurses, WOC Nurses, dieticians, surgeons, and pastoral caregivers, as well as physical and occupational therapists. A combination of experience, knowledge, patience, creativity, and persistence was needed and a large selection of products and application techniques was used to manage a constantly changing clinical situation. Planning for the transfer of care to the next setting was similarly challenging and involved considering a wide variety of clinical factors, situational variables, and financial resources.
In reflection, increasing this patient’s involvement with pouch application may have been of benefit because he became very dependent on the WOC nursing staff. However, his self-care was inhibited by his body habitus, difficulty in seeing the fistula, and the high outputs. Videotaping the pouch application process may have been helpful to post acute care providers. Earlier use of the wound management system might have facilitated transfer to the long-term care setting.
Registered Dietitians, Nurses, and Certified Diabetes Educators! Discover how to earn 2 CE hours for reading this article by visiting www.numedix.com.
1. Burch J. Priorities in nursing management of fistulas in community setting. Br J Community Nurs. 2004;6 suppl:S6–S14.
2. Pontieri-Lewis V. Management of gastrointestinal fistulas: a case study. Med Surg Nurs. 2005;14(1):68–72.
3. Colwell JC, Goldberg M, Carmel J. The state of the standard diversion. J WOCN. 2001;28(1):6–17.
4. Wainstein DE, Fernandez E, Gonzalez D, Chara O, Berkowski D. Treatment of high-output enterocutaneous fistulas with a vacuum-compaction device. A ten-year experience. World J Surg. 2008;32:430–435.
5. Gray M, Jacobson T. Are somatostatin analogues (octreotide and lanreotide) effective in promoting healing of enterocutaneus fistulas? J WOCN. 2002;29:228–233.
6. Teixeira P, Inaba K, Dubose J, et al. Enterocutaneous fistula complicating trauma laparotomy: a major resource burden. Am Surg. 2009;75(1):30–32.
7. Makhdoom ZA, Komar MJ, Still CD. Nutrition and enterocutaneous fistula. J Gastroenterol. 2003;31:195–204.
8. Evenson A, Fischer F. Current management of enterocutaneous fistula. J Gastrointestinal Surg. 2006;10:455–464.
9. Reed T, Economon D, Wiersema-Bryant L. Colocutaneous fistula management in a dehisced wound: a case study. Ostomy Wound Manage. 2006;52(4):60–66.
10. Beck D. Intestinal fistulas. The Phoenix. 2005;Winter:58–61.
11. Draus J, Huss S, Harty N, Cheadle W, Larson G. Enterocutaneous fistula: are treatments improving? Surgery. 2006;140:570–578.
12. Levy E, Frileux P, Cugnenc PH, Honinger J, Ollivier JM, Parc R. High-output external fistulas of the small bowel: management with continuous enteral nutrition. Br J Surg. 1989;76:676–679.
13. Reber CE, Roberts C, Way LW, Dunphy JE. Management of external gastrointestinal fistulas. Ann Surg. 1978;188:460–467.
14. Beck F, Rosenthal T. Prealbumin: a marker for nutritional evaluation. Am Fam Phys. 2002;65(8):1575–1579.
15. Singh R. Evaluation of nutritional status by different parameters and to predict spontaneous closure, morbidity and mortality in patients with enterocutaneous fistulas: a study of 92 cases. Int J Nutrition Wellness. 2008;6(1). Available at: www.ispub.com/journal/the.internet.journal_of_nutrition_and_wellness.html. Accessed April 4, 2009.
16. Torres AJ, Landa JI, Moreno-Azcoita M, et al. Somatostatin in the management of gastrointestinal fistulas. Arch Surg. 1992;127:97–99.
17. Sancho JJ, diCostanzo J, Nubiola P, et al. Randomized double-blind placebo-controlled trial of early octreotide in patients with postoperative enterocutaneous fistulas. Br J Surg. 1995;82:638–641.
18. Nubiola-Calonge P, Badia JM, Sancho J, Gil MJ, Segura M, Sitges-Serra A. Blind evaluation of the effect of octreotide , a somatostatin analogue, on small-bowel fistula output. Lancet. 1987;2:672–674.
19. Alvarez C, McFadden D, Reber H. Complicated enterocutaneous fistulas: failure of octreotide to improve healing. World J Surg. 2000;24:533-538.
20. Dudrick S, Mahara AR, McKelvey AA. Artificial nutritional support in patients with gastrointestinal fistulas. World J Surg. 1999;23:570–576.
21. Martinez J, Luque-de-Leon E, Mier J, Blanco-Benavides R, Robledo R. Systematic management of postoperative enterocutaneous fistulas: factors related to outcomes. World J Surg. 2008;32:436–443.
22. Rolstad B, Bryant R. Management of drain sites and fistulas. In: Bryant R (ed). Acute and Chronic Wounds, 2nd ed. St. Louis, MO; Mosby;2009:490–516.
23. Erdmann D, Drye C, Heller, L, Wong MS, Levin SL. Abdominal wall defect and enterocutaneous fistula treatment with vacuum assisted closure (VAC) system. Plast Reconstr Surg. 2001;108:2066–2068.
24. Alvarez AA, Maxwell GL, Rodriquez GC. Vacuum-assisted closure for cutaneous gastrointestinal fistula management. Gynecol Oncol. 2001;80:413–416.
25. Cro C, George KJ, Donnelly J, Irwin ST, Gardiner KR. Vacuum assisted closure system in management of enterocutaneous fistulas. Postgrad Med J. 2002;78:364–365.
26. Goverman J, Yelon J, Platz J, Singson R, Turcinovic M. The “fistula vac”: a technique for management of entercutaneous fistulas arising within an open abdomen: report of 5 cases. J Trauma. 2006;60:428–431.
27. Lloyd DA, Gabe SM, Windsor ACJ. Nutrition and management of enterocutaneous fistula. Br J Surg. 2006;93:1045–1055.













