A Laboratory Study Comparing Skin Temperature and Fluid Loss on Air-Fluidized Therapy, Low-Air-Loss, and Foam Support Surfaces
Abstract
To ensure appropriate fluid replacement, caregivers need to understand the effect of support surfaces on the rate of moisture loss from the body. A prospective study was conducted to 1) measure the rate of weight (fluid) loss on an air-fluidized therapy (AFT) surface; 2) determine the effect of bath temperature on weight loss; 3) compare weight loss and skin temperatures between foam and low-air loss (LAL) surfaces; 4) compare characteristics of individuals with high and low weight loss rates; and 5) compare weight loss rates to previously measured rates of support surface evaporative capacity.
Eight healthy adult volunteers (three men, five women, average age 33 years, average body mass index 31.0 kg/m2) participated in eight 180-minute trials (one trial per day) on a foam, an LAL, and an AFT surface at five different bath temperatures (range: ~99.0˚ F [hot] to ~88.0˚ F [low]). Weight (±10 g) was obtained before and after each trial and skin temperature (across the back) was recorded continuously. Using linear regression, weight loss rate on AFT was found to be strongly dependent upon bath temperatures: weight loss/day (g/m2-24 hours) = 53.9 x T (F) – 4030, where T is the mean skin temperature on the patient’s back (also equal to bath temperature) in (F) Fahrenheit. Using this regression equation at mid-range (94˚ F), fluid loss in an average woman (145 lb/64.5 inches/1.72 m2 body surface area [BSA]) on AFT would be estimated to be 850 g/day higher than on foam. Compared with LAL, weight loss on AFT was estimated to be 700 g/day and 800 g/day higher for the typical woman and man, respectively (P <0.05 at a mid-range bath temperature of 94˚ F). Weight loss rates varied from 480 g/m2-24 hours to 3,470 g/m2-24 hours. Weight loss and mattress evaporative rates also suggest that moisture accumulation may occur on a foam but not on an LAL or AFT surface. However, fluid intake should be increased on AFT, particularly when bath temperature settings are high.

Potential Conflicts of Interest: Dr. Lachenbruch discloses he is an employee of and owns stock in Hill-Rom. The company provided financial compensation to conduct the study and to research/write the manuscript.
Therapeutic support surfaces are known to play a role in determining patient’s fluid replacement needs.1,2 Laboratory measurements indicate that rates of moisture withdrawal — ie, the rate at which water passes across the skin and into the environment — differ substantially among surface types such as air-fluidized therapy (AFT) and standard hospital mattresses 3 and low-air-loss (LAL) and foam.4,5 Formulas for fluid replacement needs on AFT have been published6,7 but were typically developed using small numbers of patients with burns or wounds over significant areas of the body.  Two prospective studies of patients with intact skin (one based on 20 healthy subjects and 14 inpatients3 and the other based on results from a single healthy volunteer over seven consecutive days8), showed that weight loss rates on AFT depended on the bed’s bath temperature setting. However, both studies are more than 20 years old and did not measure the actual bead bath temperatures, rendering the relationship between variables somewhat unclear and worthy of fresh scrutiny.
Two prospective studies of patients with intact skin (one based on 20 healthy subjects and 14 inpatients3 and the other based on results from a single healthy volunteer over seven consecutive days8), showed that weight loss rates on AFT depended on the bed’s bath temperature setting. However, both studies are more than 20 years old and did not measure the actual bead bath temperatures, rendering the relationship between variables somewhat unclear and worthy of fresh scrutiny.
Literature Review
AFT surfaces have been shown to be effective in the management of pressure ulcers in a number of clinical trials9-12,14 and retrospective chart review studies.13 The air flow in these support surfaces is believed to assist in managing skin microclimate as well as interface pressure, shear, and friction.15-17 It also has been noted that the additional water loss associated with the airflow on AFT results in a small number of patients experiencing some degree of dehydration.9 Importantly, dehydration itself can be considered a risk factor for pressure ulcers because if blood volume is reduced, peripheral circulation may be impaired, decreasing nutrient and oxygen supply to tissues.18

Fluid loss in patients with intact skin. When a patient is on a support surface, moisture can be lost from the body via several pathways. In addition to losses via urine and feces, water is lost through intact skin by diffusion (transepidermal water loss) and transpiration.
TEWL. TEWL is the diffusion of H2O molecules across the epidermis from the moist, vascular dermis into the environment. TEWL rate is determined by the resistance to diffusion imposed by the epidermis (which serves as a barrier to water loss) and the concentration of water molecules on each side of the epidermis. Because the fluid concentration generally is higher in the tissue than in the external environment, some outflow due to TEWL typically exists and, like diffusion generally, has been shown to be strongly temperature-dependent.19 The support surface influences TEWL by affecting skin temperature; due to the airflow, it affects the downstream concentration of H2O molecules as they diffuse outward. Support surfaces that withdraw water molecules from the skin/support surface interface rapidly (eg, AFT) would be expected to increase TEWL over a surface that removes H2O from the surface more slowly. TEWL is normally a relatively minor component of moisture loss overall, with estimates ranging from approximately 100 to 600 g/m2-24 hours.19-22
Perspiration. The moisture produced by the sweat glands, driven primarily by controls in the thermoregulatory system, is unrelated to the concentration of H2O molecules at the surface. Validated models23-26 have been developed to estimate the amount of general and local perspiration produced by the body under a variety of conditions. Because local skin temperature is one of the factors that has been shown to affect the rate of local perspiration23-25,27 and surfaces have been shown to affect skin temperature,28 it is reasonable to expect that support surfaces influence the rate of perspiration.
Respiratory losses. In addition to moisture loss from intact skin, H2O loss from the lungs has been estimated to account for one third of overall fluid losses under resting conditions.29
Fluid loss from non-intact skin. Other mechanisms may come into play when significant areas of the skin are absent, as in the case of a burn patient.
Exudate from open wounds. When the skin barrier is not present, fluid is free to drain directly to the surface. The quantity of fluid from uncovered wounds has been found to depend on the age and depth of the wound but has been measured in the early stages at approximately 0.81 g-cm2/day of wound area (8,100 g/m2 over 24 hours).6
Evaporation of fluid from open wounds. Fluid that has drained from the wound is exposed directly to the evaporative conditions of the environment, which are determined in large part by the support surface. Measured support surface evaporative capacities — ie, their ability to remove moisture already present on the surface — have been found to range from approximately 30 g/m2-24 hours for foam surfaces5,15 to 1,500 to 3,000 g/m2-24 hours for LAL surfaces5,15 to 16,800 g/m2-24 hours for AFT.15 It is essential to emphasize that the actual moisture removal that occurs under conditions in which the skin is intact may be only a very small percentage of what the surface is capable of evaporating because the skin barrier prevents the free flow of moisture to the surface. In other words, unless the skin is not present, the actual amount of moisture evaporated may be notably less than the measured evaporative capacity of the surface.
Weight loss on support surfaces. When skin is intact, the support surface 1) affects skin temperature, which drives both the sweat response and TEWL through the temperature’s effect on the rate of diffusion and 2) influences the rate of H2O removal from the skin surface, which by lowering the downstream concentration of H2O molecules determines the rate of outward diffusion through the skin (TEWL) — ie, the rate of moisture accumulation on the skin. The combined effects of the above determine the rate at which moisture is brought to the surface and lost from the body.
Weight loss on AFT. The most thorough investigation of weight loss on AFT involving patients with intact skin was the study previously noted by McNabb and Hyatt.3 Using linear regression, two equations were developed:
1. Healthy volunteers: Weight loss/day (g/m2-24 hours) = 53.5 x T (F) – 4090
2. Inpatients: Weight loss/day (g/m2-24 hrs) = 56.9 x T (F) – 4340, where T(F) = bath temperature in degrees Fahrenheit.
As mentioned, McNabb and Hyatt did not actually measure bath or skin temperature; instead, they recorded the bed’s temperature setting. Actual bath temperatures depend on both the bed temperature setting and the ambient temperature. Further, all studies on inpatients were conducted at settings of 88˚ F to 92˚ F and studies on healthy volunteers were conducted only at settings of 86˚ F to 94˚ F; therefore, their regression equations are based on data points that cover only the lower half of the range of selectable bath temperatures, which range from 84˚ to 102˚ F.
McNabb and Hyatt reported that the weight loss on a standard hospital mattress among healthy volunteers was 480 g/m2-24 hour, approximately equal to the mean of 520 g/m2-24 hours they observed on AFT at the lowest temperature setting (86˚ F). No measurements were reported on LAL surfaces. A previous laboratory study conducted by Michaels and Sorensen8 in which weight loss was measured for seven consecutive days on a single healthy subject predicted a loss rate of 1,524 g/m2-24 hours at a bath temperature of 96.8˚ F, 40% higher than the weight loss predicted by the McNabb and Hyatt regression at this temperature. McNabb and Hyatt noted that the Michaels and Sorensen study was conducted at a very high ambient temperature of 84.2˚ F, further compromising comparison or generalization of their results.
The preceding estimates of moisture withdrawal on AFT from intact skin can be compared to measurements of daily fluid loss on burned areas where the skin is not present, which range from approximately 3,000 to 8,100 g/m2.6,7 This is three to eight times greater than the McNabb and Hyatt estimate for intact skin of approximately 1,000 g/m2-24 hours at mid- range bath temperatures.
In summary, studies to guide fluid replacement needs on AFT are limited and those available suggest a strong dependence on bath temperature and wound area. The only large-scale study based on individuals with intact skin did not record skin temperatures and did not take measurements in the upper half of the selectable temperature range. The McNabb and Hyatt equations appear to be the only formulas available to predict weight loss on AFT for persons with intact skin but study age and procedures suggest a need for scrutiny. Further, no data sets were found that present these results in comparison with standard foam and LAL surfaces. Thus, the current study was conducted to: 1) measure the rate of weight loss through intact skin on an AFT surface using non-urinary, non-fecal weight loss exclusively — ie, perspiration, TEWL, and respiratory losses; 2) determine the degree to which this rate depends on temperature; 3) compare resultant data with the rates of weight loss on a typical foam and LAL surface; 4) determine if any significant differences exist with respect to age, weight, body mass index (BMI), or gender between volunteers with high versus low loss rates; and 5) compare the rates of weight loss with the measure evaporative capacities of each surface.

Methods
Eight healthy adult volunteers (three men, five women ranging in age from 26 to 55 years) without open wounds were recruited from the community. Participants were selected to achieve a rough gender balance and to cover a relatively broad range of weight (115 to 290 lb) and BMI (20.4 to 48.3 kg/m2). To measure non-urinary, non-fecal weight loss — ie, perspiration, TEWL, and respiratory losses — on subjects whose skin is intact, each subject completed 3-hour trials on a foam surface, a LAL surface with a highly vapor-permeable cover, and the same LAL surface with a moderately vapor-permeable cover. Each subject also completed five trials on an AFT surface at five different temperature settings.  Equipment. Support surfaces used in this study were all manufactured by Hill-Rom (Batesville, IN) and included Clinitron® C-II air-fluidized therapy surface; Totalcare SpO2rt® Plus low-air-loss surface with Lamcotec Nylon Highly vapor- permeable ticking; Totalcare SpO2rt® Plus low air-loss surface with Dartex moderately vapor-permeable ticking; and NP100 foam surface.
Equipment. Support surfaces used in this study were all manufactured by Hill-Rom (Batesville, IN) and included Clinitron® C-II air-fluidized therapy surface; Totalcare SpO2rt® Plus low-air-loss surface with Lamcotec Nylon Highly vapor- permeable ticking; Totalcare SpO2rt® Plus low air-loss surface with Dartex moderately vapor-permeable ticking; and NP100 foam surface.
Volunteers. Eight healthy adults (three men, five women, average age 33 years, average BMI 31.0 kg/m2) volunteered for the study (see Table 1). IRB approval for this type of laboratory study (healthy subjects lying on beds) was interpreted from guidelines to not be required.
Procedures. All volunteers completed eight 180-minute trials on various surfaces and at different bath temperature settings (see Table 2). Only one trial was performed on a given day and tests were typically separated by a 24-hour period unless weekends intervened. All volunteers were clothed in underwear and a hospital gown for testing. Before each trial, the volunteer was weighed to the nearest 10 g on a precision balance scale (Adams 330 x 0.02 lb Precision Platform Scale, American Weigh Scales, Irvine, CA). The balance was calibrated at the factory and checked with calibration weights when it arrived at the laboratory at the start of the study. All trials were started with volunteers in a comfortable state of hydration. Participants were not allowed to drink or use the rest room between the initial and final weight measurements.
Four Type K (chromel-alumel) thermocouples were attached to the underside of the body on the upper and lower back to measure overall back temperature in contact with the support surface. Additional thermocouples were positioned to measure bath and room temperature. Temperatures were collected and stored at a rate of one sample/second using a Hydra Data logger.
 AFT trials. Volunteers were positioned in the supine position directly on the AFT filter sheet and covered with a single cotton sheet. Each participant was tested at five bead bath temperatures — high: highest bath temperature setting; low: lowest bath temperature setting; medium: midway between the high and low settings; medium high; and medium low — the latter two settings midway between the medium and extreme temperature settings.
AFT trials. Volunteers were positioned in the supine position directly on the AFT filter sheet and covered with a single cotton sheet. Each participant was tested at five bead bath temperatures — high: highest bath temperature setting; low: lowest bath temperature setting; medium: midway between the high and low settings; medium high; and medium low — the latter two settings midway between the medium and extreme temperature settings.
LAL and foam trials. Each participant also completed a single trial on the two LAL surfaces and the foam surface. The participant was placed in the supine position directly on the support surface and covered with a single moderate weight blanket and a cotton sheet to simulate actual use conditions.
At the conclusion of each trial, the subject exited the bed and was immediately weighed to the nearest 10 g (0.02 lb).
Comparison of observed weight loss with evaporative capacities. The observed rates of weight loss were compared with previously collected and published laboratory measurements of the surfaces’ ability to evaporate moisture. The evaporative capacity is the rate at which water can be evaporated from a surface given an infinite supply. Evaporative capacity data were calculated from evaporative resistance values reported by VanGilder and Lachenbruch15 using the method of Nicholson et al.32
Data analysis. All data were collected using a Fluke Hydra Data Logger (Everett, WA), downloaded to a PC, and analyzed using the Analysis Tool Pack in Microsoft Excel 2007. Body surface area (BSA) (m2) of each subject was calculated using the Mosteller formula30:  Using the BSA, the rate of weight lost over each trial was normalized for presentation in g/m2-24 hours. The relationship between skin temperature and the rate of weight loss on AFT was determined by linear regression with α = 0.05. Weight loss rates on AFT and other surfaces were compared using t-tests (where α = 0.05). The comparison between mean BMI, age, and weight among subjects with the highest and lowest rates of weight loss also were compared using t-tests using α = 0.05.
Using the BSA, the rate of weight lost over each trial was normalized for presentation in g/m2-24 hours. The relationship between skin temperature and the rate of weight loss on AFT was determined by linear regression with α = 0.05. Weight loss rates on AFT and other surfaces were compared using t-tests (where α = 0.05). The comparison between mean BMI, age, and weight among subjects with the highest and lowest rates of weight loss also were compared using t-tests using α = 0.05.
Results
Rate of weight loss on AFT surface. The mean rate of weight loss (as an indicator of non-urinary, non-fecal moisture loss) on AFT over all trials was 1,032 g/m2-24 hours (SD = 655). Mean bath and low back temperature over these 40 trials was 93.8˚ F, which was very close to the midpoint of the selectable range on the product of 93.0˚ F.
Relationship between bath temperature and weight loss on AFT. The relationship between AFT bath temperature and 24-hour weight loss showed a mean increase in weight loss rate of approximately 54 g/m2-24 hours for each degree F increase in bath temperature. The regression slope is significant at the α = 0.05 level (P = 0.033; R2 = 0.114); 24-hour weight loss can be described by the regression equation: Rate of Weight Loss (g/m2-24 hrs = 53.9 x T(F) – 4030, where T(F) = bath or back skin temperature (Fahrenheit) (see Figure 1).
Comparison between weight loss on AFT and other surfaces. Mean 24-hour weight loss rate on AFT at the high temperature setting (1,535 g/m2-24 hours; SD = 987; mean bath temperature = 98.95˚ F) was significantly higher than on the LAL surface (640 g/m2-24 hours; SD = 235; P = 0.01) or the foam surface (543 g/m2-24 hours; SD = 238; P = 0.02). Mean weight loss rate on AFT at the medium high temperature setting (1,067 g/m2-24 hrs; SD = 567; mean bath temperature = 97.24˚ F) was significantly higher than on LAL surface (P = 0.03) or the foam surface (P = 0.03). At each of the three lower bath temperature settings (medium, medium low, and low), the weight loss rate on AFT was equivalent to both foam and LAL. Mean loss rate on AFT when all five temperature settings were pooled (1,032 g/m2-24 hours; SD = 655) was significantly greater than LAL (P = 0.04) or foam (P = 0.04) (see Table 3).
At the mid-range setting of 94˚ F, losses for the average female participant (145 lb/ 64.5 inches/1.72 m2 BSA) on AFT were estimated to be 850 g/day higher than on foam. For a typical male participant (180 lb/ 69.5 inches /2.00 m2 BSA), losses were estimated to be approximately 1,200 g/day greater than on foam. Compared with LAL, weight loss on AFT was estimated to be 700 g/day and 800 g/day higher for the typical female and male, respectively (see Table 4).
The effects of age, weight, BMI, and gender on rate of weight loss. On AFT, large inter-individual differences were observed in the overall rates of weight loss and in the degree to which weight loss was affected by temperature. Four participant responses were nearly identical and showed relatively low mean weight losses and limited increase in weight loss with temperature. Four others had much higher mean loss rates and more rapid increases with temperature. The mean weight loss rate at a bath temperature of 94˚ F varied by a factor of nearly three between participants between the lowest (650 g/m2-24 hours) and highest rates (1,817 g/m2-24 hours) of weight loss (see Figure 2). The dependence of weight loss on temperature also varied widely as indicated by the variation in the slopes of the 24-hour weight loss rate versus bath temperature regression lines. These slopes ranged from negative 6.0 g/m2-24 hours per degree F to 164.8 g/m2-24 hours per degree F (see Table 5).
Mean weight loss differences among the four participants with the highest (mean 1,413 g/m2-24 hours, SD = 325) and the four with the lowest rate (mean = 644 g/m2-24 hours, SD = 28) were significant (P = 0.014) but demographic variables did not differ between these two groups (see Table 6).
Differences between volunteers with the lowest weight loss sensitivity to temperature (mean = 15.1 g/m2-24 hours per degree F, SD = 16.1) compared to those with the greatest sensitivity (mean = 91.0 g/m2-24 hours per degree F, SD = 65.2) were not significant (P = 0.059). Demographic variables also did not differ significantly at the α = 0.05 level between these two groups (see Table 7).
Comparison of surface evaporative capacity to observed weight loss. Weight lost on the foam mattress was greater than the previously measured evaporative capacity for foam, suggesting a tendency for moisture to accumulate on the skin. On the LAL product, the rates of weight loss from the body and the capacity of the surface were approximately equal, suggesting little or no accumulation on the skin. On AFT, the rates of weight loss were only 4% to 7% of the measured surface capacity to evaporate moisture on the surface (see Table 8).

Discussion
Weight loss on AFT. The rates of weight loss observed in the present study are consistent with those observed by McNabb and Hyatt.3 At a bath temperature of 96.8˚ F, current study data predict weight loss of 1,191 g/m2-24 hours for healthy adults, compared to McNabb and Hyatt’s predicted loss rates at the same temperature of 1,088 g/m2-24 hours for healthy adults (-8.8%) and 1,164 g/m2-24 hours for inpatients (-2.2%). Although inpatients were not included in the present study, the observation that losses among inpatients and healthy volunteers were very similar in the McNabb and Hyatt study supports generalization of the new results to the patient population.
At 95.8˚ F, Michaels and Sorensen8 reported a weight loss of 1,534 g/m2-24 hours, 30% to 40% higher than either of the McNabb and Hyatt Equations or those presented in the current study. As noted previously, the fact that Michaels and Sorensen conducted their tests at an ambient temperature of 84˚ F suggests additional thermal stress and perspiration beyond what might be expected for the temperature setting alone.
Additionally, Michaels and Sorensen’s measurements were limited to a single individual who was studied over seven consecutive days. The high degree of variation between subjects observed in the current study suggests that generalization of measurements obtained on any given individual is problematic.
Current study results can be used to guide fluid replacement for patients on AFT and other surfaces. Ayello et al1 recommended that patients on AFT, as compared to a standard mattress, receive an extra 10 to 15 mL of fluid daily per kg. The losses presented here are consistent with but broaden this range. Using the values in Table 4, daily fluid loss on AFT versus foam for the average female participant increases from 7.2 mL/kg body weight at a bath temperature of 90˚ F to 18.3 mL/kg at 98˚ F. For the typical male participant, the additional intake requirements would be 6.9 mL/kg at 90˚ F and 30.7 mL/kg at 98˚ F.
Temperature dependence of weight loss on AFT. The observed increase in weight loss per degree F of 53.9 g/m2-24 hours per degree F was equivalent to the values obtained by McNabb and Hyatt of 53.5 for healthy volunteers and 56.9 g/m2-24 hours per degree F for inpatients. (one-sided t-tests; P = 0.98 versus healthy volunteers; P = 0.87 versus inpatients). This suggests that despite the differences in test methods any of the three equations should provide reasonable results throughout the potential range of bath temperatures.
The mean weight loss rates (see Table 4) can be used to estimate the fluid replacement needs for average-sized male and female patients placed on foam, LAL, and AFT support surfaces. Alternatively, one can combine the regression equation obtained for weight loss on AFT from Figure 1 with the Mosteller formula for BSA (equation in Methods section) to obtain a general formula to estimate the rate of weight loss per day as a function of height and weight.
Weight loss on AFT versus other surface types. McNabb and Hyatt reported a weight loss of 480 g/m2-24 hours on a standard hospital mattress. This was equivalent to the mean reported here (543 g/m2-24 hours; SD = 198) (one-sided t-test; P = 0.38). The relationship between the measured rates of weight loss on the standard hospital mattress and the predicted rate on AFT also was consistent on both studies. McNabb and Hyatt’s equation for healthy volunteers predicts a doubling to 960 g/m2-24 hours at a temperature of 94.5˚ F; the current results suggest a doubling at 94.8˚ F.
Variation in weight loss rates among individuals. One of the primary purposes of this study was to determine if the ability to estimate weight loss on AFT could be enhanced by using variables other than bath temperature, as McNabb and Hyatt had done. This appears to have been the first study to do so. Although the four individuals who lost the most weight did not differ significantly from those who lost the least with respect to gender, weight, age, or BMI, identifying the sources of the broad differences between individuals appears to be a valuable topic for future study.
Comparison of surface evaporative capacity to observed weight loss. It should be emphasized that the evaporative capacity of a surface is its ability to evaporate an infinite moisture supply. Skin is a natural barrier to fluid loss; therefore, if the skin is intact, moisture will not be lost from the body at the full evaporative capacity but at some reduced level that is modified by the rate at which moisture passes from the interior to the surface of the skin from which it can be evaporated.
 When the weight loss is appreciably higher than surface’s evaporative capacity, moisture will accumulate on the skin, which can cause the skin to weaken,33 making it notably more susceptible to breakdown.34,35 Conversely, if the weight loss is equal to or less than the evaporative capacity, the moisture should not accumulate on the skin. Three important observations were noted from the study data:
When the weight loss is appreciably higher than surface’s evaporative capacity, moisture will accumulate on the skin, which can cause the skin to weaken,33 making it notably more susceptible to breakdown.34,35 Conversely, if the weight loss is equal to or less than the evaporative capacity, the moisture should not accumulate on the skin. Three important observations were noted from the study data:
1. Weight lost from the body on the foam mattress is greater than the evaporative capacity, suggesting a tendency for moisture to accumulate on the skin. This is consistent with the findings from Flam,28 who measured the rate of moisture accumulation on the backs of hospital patients on different support surface types and found high rates of accumulation on foam (30.1 g/m2-hour);
2. The rates of weight loss from the body and the evaporative capacity of the LAL surface are approximately equal, suggesting little or no accumulation on the skin. This also is consistent with Flam’s data, in which the LAL surfaces tested accumulated moisture on the skin at approximately 3.8 g/m2-hour;
3. The rates of weight loss on AFT were only 4% to 7% of the measured surface capacity to evaporate moisture on the surface. This indicates that the weight loss through the skin on AFT is limited by the supply of moisture brought to the surface — perspiration and TEWL — and not by the capacity of the surface to evaporate moisture (see Table 8).
Future Studies
Although weight loss on AFT is strongly temperature-dependent, the variation between participants suggests other patient-related factors may enhance prediction accuracy. No significant differences were found between high-loss and low-loss participants with respect to age, weight, BMI, and gender, but re-examining these variables and others with future studies involving larger sample sizes and a broader age range could be of value.

Limitations
The primary limitation of this study is that it involved a relatively small sample of eight persons. This may be important because one of the observations was the high degree of variation in moisture loss among individuals Additionally, the persons tested were healthy volunteers who may respond differently than the actual patient population likely to be placed on these surfaces.
Conclusion
Results of a laboratory study to compare weight loss as an index of fluid loss on various support surfaces suggest that fluid replacement needs of patients on AFT with intact skin are strongly influenced by bath temperature. Mean losses on AFT were found to be greater than losses on foam at all temperature settings, although due to high variation between subjects, the differences were significant at only the two highest temperature settings. At lower study temperature settings, mean losses on AFT were 57% greater than on foam. At the two highest settings, losses on AFT were 100% and 180% greater than on foam, respectively. In addition, mean losses on AFT were significantly greater than on LAL at only the highest temperature settings; at the lower settings, mean losses on AFT were 33% greater than on LAL. At the higher settings, losses on AFT were 66% and 140% greater than on LAL, respectively. The following regression equation can be used to estimate the rate of weight on loss on AFT for patients with intact skin.
Last, although no significant differences with respect to age, weight, BMI, or gender were found among study participants identified as “high-loss” and “low-loss,” the magnitude of weight loss varied greatly among study volunteers, 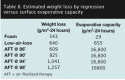 suggesting that use of any predictive formulas should be accompanied by regular and frequent patient weight measurements.
suggesting that use of any predictive formulas should be accompanied by regular and frequent patient weight measurements.
Acknowledgments
The author thanks Rachel Williamson and Josh Williams for their laboratory work on this project and Catherine VanGilder for her contributions in the review process.
Dr. Lachenbruch is a Senior Biomedical Engineering Specialist, Surface Research and Development, Hill-Rom, Batesville, IN. Please address correspondence to: Charles Lachenbruch, PhD, 110 Squires Drive, Lakeway, TX 78734; email: Charlie.Lachenbruch@Hill-Rom.com.
1. Ayello EA, Thomas DR, Litchford MA. Nutritional aspects of wound healing. Home Healthcare Nurs. 1999;17(11):719–729.
2. Kondracki N, Collins S. The importance of adequate hydration. Ostomy Wound Manage. 2009:55(12):16–20.
3. McNabb LJ, Hyatt J. Effect of an air-fluidized bed on insensible water loss. Crit Care Med. 1987;15(2):161–162.
4. Reger S, Adams T, Maklebust J, Sahgal V. Validation test for climate control on air-loss supports. Arch Phys Med Rehabil. 2001;82:597–603.
5. Figliola RS. A proposed method for quantifying low-air-loss mattress performance by moisture transport. Ostomy Wound Manage. 2003:49(1):32–42.
6. Michaels J, Sorensen B. Water and sodium balance: the effect of the air-fluidized bed on burned patients. Burns. 1983;9(5):305–311.
7. Davies JW, Lamke LO, Liljedahl SO. A guide to the rate of non-renal water loss from patients with burns. Br J Plast Reconstr Surg. 1974:27(4):325–329.
8. Michaels J, Sorensen B. The physiology of a healthy person in the air-fluidized bed. Burns. 1983;9:158–168.
9. Allman RM, Walker JM, Hart MK, LaPrade CA, Noel LB, Smith CR. Air-fluidized beds or conventional therapy for pressure sores. Ann Int Med. 1987;107(5):641–648.
10. Strauss MJ, Gong J, Gary BD, Kalsbeek WD, Spear S. The cost of home air-fluidized therapy for pressure sores. J Fam Pract. 1991;33(1):52–59.
11. Bristow JV, Goldfarb EH, Green M. Clinitron therapy: is it effective? Geriatric Nurs. 1987;8(3):120–124.
12. Munro BH, Brown L, Heitman BB. Pressure ulcers: one bed or another? Geriatric Nurs. 1989;10(4):190–192.
13. Ochs RF, Horn SD, van Rijswijk L, Pietsch CA, Smout RJ. Comparison of air-fluidized therapy with other support surfaces used to treat pressure ulcers in nursing home residents. Ostomy Wound Manage. 2005;51(2):38–68.
14. Jackson BS, Chagares R, Nee N, Freeman K. The effects of a therapeutic bed on pressure ulcers: an experimental study. J Enterostom Ther. 1988;15(6):220–226.
15. VanGilder C, Lachenbruch C. Air fluidized therapy: physical properties and clinical uses. Ann Plast Surg. In press.
16. Newsome MW, Johns LA, Pruitt BA. Use of an air-fluidized bed in the care of patients with extensive burns. Am J Surg. 1972;124(1):52–56.
17. Reger S, Ranganathan V, Sahgal V. Support surface interface pressure, microenvironment, and the prevalence of pressure ulcers: an analysis of the literature. Ostomy Wound Manage. 2007;53(10):50–58.
18. Ferguson M, Cook A, Rimmasch H, Bender S, Voss A. Pressure ulcer management: the importance of nutrition. MEDSURG Nurs. 2000;9(4):163–175; quiz, 176–177.
19. Grice K, Sattar H, Sharrat M, Baker H. Skin temperature and transepidermal water loss. J Investig Dermatol. 1971;57(2):108–110.
20. Blank I. Transport into and within the skin. Br J Dermatol. 1969;81(suppl 4):4–10.
21. Bullard RW, Banerjee MR, Chen F, Elizondo R, MacIntyre BA. Skin temperature and thermoregulatory sweating: a control systems approach. In: Hardy JD, Gagge AP, Stolwijk JA (eds). Physiological and Behavioral Temperature Regulation. Springfield, Illinois, Charles C. Thomas, Publisher,1970:597– 610.
22. Brandeis K. Fluid physiology: an online text. Available at: www.anaesthesiaMCQ.com. Accessed July 23, 2010.
23. Frank S, Raja S, Bulcao C, Goldstein D. Relative contribution of core and cutaneous temperature to thermal comfort and autonomic responses in humans. J Appl Physiol. 1999:86(5):1588–1593.














