Applying Observations from Forensic Science to Understanding the Development of Pressure Ulcers
The prevention and management of pressure ulcers has become increasingly important in the care of patients, the accreditation of hospitals, and more recently, in hospital and skilled nursing facility (SNF) litigation. Many aspects of documentation, including admission and progressive photographs, comprise a well-rounded pressure ulcer management program; they become vital should questions about care arise. Succinct staging and treatment entries on the medical record also are essential to hospital nursing educators and policymakers, as well as to those reviewing charts for data and litigation purposes.  Recently, controversy over the staging of reddened areas and classification of deep tissue pressure injury has increased. The working definition of deep tissue injury for this paper is “a pressure-related injury to subcutaneous tissues under intact skin. Initially these lesions have the appearance of a deep bruise and they may herald the development of subsequent development of a Stage III to Stage IV pressure ulcer, even with optimal treatment.”1 The various staging systems imply that pressure ulcers progress from Stage I to Stage IV over a period of time if measures are not taken to provide pressure relief – ie, they take a long time to develop. This apparently is not the case with deep tissue injuries. Slow progression to Stage IV occasionally occurs when pressure-related diabetic foot ulcers incur repetitive low-level insults with each step in patients who are not taking appropriate foot protection precautions, who may not understand the need for offloading, or whose caregivers are unfamiliar with the necessary observational and positioning skills. However, in an environment where staff are trained and knowledgeable regarding pressure ulcers, the appearance of a pressure-related skin dyscrasia usually triggers immediate action and pressure-relief measures are adjusted to accommodate skin alteration. Most Stage II ulcers and deep tissue injuries (precursors of Stage III and IV ulcers) occur as a single insult; the final “product” is dependent on the amount of pressure per square inch multiplied by the length of time of sustained compression and the vulnerability of the patient (particularly exhibited during periods of hypotension).2,3 A recent review article4 states that shear has been shown to accelerate the entire process.
Recently, controversy over the staging of reddened areas and classification of deep tissue pressure injury has increased. The working definition of deep tissue injury for this paper is “a pressure-related injury to subcutaneous tissues under intact skin. Initially these lesions have the appearance of a deep bruise and they may herald the development of subsequent development of a Stage III to Stage IV pressure ulcer, even with optimal treatment.”1 The various staging systems imply that pressure ulcers progress from Stage I to Stage IV over a period of time if measures are not taken to provide pressure relief – ie, they take a long time to develop. This apparently is not the case with deep tissue injuries. Slow progression to Stage IV occasionally occurs when pressure-related diabetic foot ulcers incur repetitive low-level insults with each step in patients who are not taking appropriate foot protection precautions, who may not understand the need for offloading, or whose caregivers are unfamiliar with the necessary observational and positioning skills. However, in an environment where staff are trained and knowledgeable regarding pressure ulcers, the appearance of a pressure-related skin dyscrasia usually triggers immediate action and pressure-relief measures are adjusted to accommodate skin alteration. Most Stage II ulcers and deep tissue injuries (precursors of Stage III and IV ulcers) occur as a single insult; the final “product” is dependent on the amount of pressure per square inch multiplied by the length of time of sustained compression and the vulnerability of the patient (particularly exhibited during periods of hypotension).2,3 A recent review article4 states that shear has been shown to accelerate the entire process.
In a recent review of the literature and various staging systems, Ankrom et al5 conclude that the terminology and staging systems developed up to now do not consider deep tissue pressure damage under intact skin and that the issue needs to be clarified. They propose that the entire issue of reddened areas needs to be addressed and that a consistent method of differentiation and diagnosis needs to be developed. Shortly after their article was published, the National Database for Nursing Quality Improvement (NDNQI)6 reviewed the conference report from the National Pressure Ulcer Advisory Panel’s (NPUAP)29 consensus efforts reporting that the Panel had questions about the entire validity of staging. [Ed. Note: Subsequently, the NPUAP developed a new staging system that includes deep tissue injury that is available at www.npuap.org.]
Studying autopsies and pathology findings can enhance understanding of processes that occur in living persons. Hence, research performed on decedents by forensic pathologists – cataloging visible changes that occur in skin that reflect the dead and decaying flesh underneath – is of benefit to wound care clinicians from all disciplines. Furthermore, many animal studies provide plausible explanations for the suddenness of deep tissue injury and increase knowledge of the demarcation unique to isolated necrosis in living persons and warm-blooded animals. The purpose of this descriptive article, which assimilates the author’s clinical observations regarding deep tissue injuries with forensic research, including compilations of international collaboration and corroboration as well as information from the literature, clinical reports, expert opinions, and interpretations of events, is to provide a foundation for the development of a theoretical temporal framework describing the characteristics of deep tissue injuries up to 14 days after the initial insult. This, in turn, will provide a basis for further research into this complex and disturbing phenomena.
Standardizing Terminology for Stage I Pressure Ulcers
The first widely applied classification for pressure ulcers was the Shea system of staging.7 When the NPUAP revised the four-stage staging system to be included in the Agency for Health Care Policy and Research (AHCPR) guidelines8 published in 1992, changes were made to the definition of a Stage I pressure lesion, describing it as “…an area of non-blanching erythema, persistent redness with the skin still intact.” The descriptions of the other stages also were enhanced to further enable clinicians at the bedside. This system has aided clinicians for decades, facilitating establishment of guidelines and protocols for pressure ulcer prevention and treatment. In 1998, the NPUAP revised the definition of Stage I pressure ulcers to include a description of skin damage in dark-skinned people.9
Diagnosing a Stage I pressure ulcer continues to be a challenge for clinicians.10 Before the NPUAP statement on this issue, “blanching” was considered the single most compelling characteristic of a Stage I ulcer, along with intact skin (see Figure 1). The presence of blanching indicated an area of vulnerability but not injury. If at that point pressure relief measures were reinforced, the Stage I pressure area would resolve with no other intervention, usually within 2 to 3 days.11 Studies are needed to ascertain the predictability of the Stage I classifications, particularly regarding “blanchable” reddened areas with capillary refill present, to determine if and when these lesions resolve. However, healthcare databases will frequently present statistics “with Stage I” and “without Stage I”,12 seemingly indicating that the prevailing attitude in the US (and internationally in some cases) is that Stage I ulcers are superficial and self-resolving. 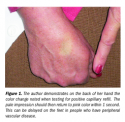 The entire staging process infers a progressive scale, with Stage I the least and Stage IV the most invasive. However, when blanching (the initial phase in testing for capillary refill) is considered, the vascular literature reflects that absence of blanching, or lack of capillary refill, is tantamount to critical ischemia of the tissues, absence of perfusion, and tissue death.13-17 Recognizing a Stage I ulcer versus deep tissue pressure injury is important, especially at the time of admission to a facility or service. The development of a Stage IV pressure ulcer on an inpatient or resident has become cause for litigation in hospitals, SNFs, and home care agencies.18-25
The entire staging process infers a progressive scale, with Stage I the least and Stage IV the most invasive. However, when blanching (the initial phase in testing for capillary refill) is considered, the vascular literature reflects that absence of blanching, or lack of capillary refill, is tantamount to critical ischemia of the tissues, absence of perfusion, and tissue death.13-17 Recognizing a Stage I ulcer versus deep tissue pressure injury is important, especially at the time of admission to a facility or service. The development of a Stage IV pressure ulcer on an inpatient or resident has become cause for litigation in hospitals, SNFs, and home care agencies.18-25
To make accurate early diagnoses, clinicians must understand that the skin is contiguous with and gets its blood supply from the underlying layers of tissues. It reflects deeper vascular events and is also affected by systemic conditions in the body as a whole. Broken or blistered skin is no longer a Stage I ulcer. However, the reverse is not necessarily true. Intactness should not be synonymous with a Stage I ulcer. In vivo animal research26,27 indicates that skin is the last layer of tissue to lose viability under prolonged pressure and can be nonviable and still not be broken for up to 14 days. The tensile strength of skin rivals that of connective tissue and ligaments, a phenomenon frequently noted in deep tissue pressure damage and documented by forensic pathologists in corpses.
Aturaliya and Lukasewycz28 state, “Skin is the last of a mummified body’s tissue to desiccate…accounting for its [presence] in body areas of little underlying tissue between bone and skin.” The bony prominences these authors describe are the most common areas for pressure lesions. Within this conceptual framework, it becomes apparent that capillary refill should be present in a Stage I lesion. It helps discern areas of hyperemia that signify damage that occurs as a result of “no-flow”/”re-flow” injuries.29 These injuries “prime” the microcirculation for that final pressure insult that results in a deep tissue injury. In vivo animal studies30-33 indicate that in episodes of hypovolemia with subsequent expansion of the intravascular compartment to replenish blood volume, or where arterial insufficiency results in an area of ischemia and then circulation is re-established, or when pressure compresses capillaries and then is relieved, sudden reperfusion creates an additional injury. This tissue injury increases with each ischemia-reperfusion cycle, the duration of ischemia, and frequency of ischemia-reperfusion cycles. These same animal studies indicate that the repetition of the ischemia-reperfusion cycle inflicts more microcirculation damage than single prolonged ischemic insults. This also indicates that an area of hyperemia with positive capillary refill can quickly convert to a deep tissue injury if offloading is inadequate or if offloading is adequate one minute but a sudden drop in blood pressure or an embolus to the arterial supply (such as to the lower extremity) occurs. If the clinician is waiting to see a non-blanching lesion before recognizing it as a documentation-worthy Stage I ulcer, the opportunity to prevent irreversible tissue damage will have been missed.
Approaching staging from this perspective enables the clinician to accurately assess the patient at any point along the continuum; it is particularly crucial at admission. Missing a diagnosis of deep tissue injury on admission can result in the follow-up identification and documentation of an advanced stage nosocomial pressure ulcer 10 to 14 days into the patient’s stay. Not only does this impact prevalence studies and quality improvement tracking for the facility, but it also can be the source of great anguish and anger on the part of patients and families, resulting in unnecessary lawsuits. Clarifying the difference between a Stage I pressure ulcer and “reddened areas” that are early manifestations of actual or suspected deep tissue injuries may prevent many of these lawsuits.34 Photographs of all reddened areas – as taken of open lesions, blisters, eschars, and the like to gather evidence, identify discolorations indicative of deep injuries, and compile a basis for evidence-based practice – are essential.
To clarify subsequent terminology, capillary refill will be used in lieu of blanching because it best describes the phenomena observed. The physiology of tissue death must be examined and described in clear clinical terms so the perception of the event is viewed by all disciplines according to the same standards.
Legal Concerns in the Diagnosis of Pressure Ulcer Stages
Not all pressure ulcers are preventable. In conjunction with this position, legal advisors to hospitals and SNFs recommend avoiding use of terms such as prevention and injuries in formulating written policies and treatment protocols.35 Terms such as pressure-relief measures, ulcers, lesions, or damage are perceived as more accurate and neutral, extracting blame and intention or negligence from the equation. These are helpful first steps to resolving controversies over staging and events leading up to the development of a pressure ulcer. If a pure science and evidence-based practice is to emerge for the diagnosis and management of pressure ulcers, etiologies and tissue response have to be delineated.36 The knowledge and expertise of wound care specialists must be translated into practical approaches that can be used by all clinicians. Guidelines, protocols, risk scales, assessment forms, and state-of-the-art equipment and treatments have come a long way in improving bedside care and lowering prevalence rates of nosocomial pressure ulcers. However, national benchmarking over the past few years has shown that nosocomial pressure ulcer rates have leveled at about 8% (including Stage I ulcers) and 4.1% (excluding Stage I ulcers).12,37 Although other explanations may exist, this strongly suggests that some ulcers are not preventable. However, until all the events leading up to irreversible tissue injuries can be didactically presented and detailed predictors of deeper Stage III and Stage IV pressure ulcers can be pinpointed, the truly unpreventable ulcers cannot be identified. Conversely, once wound care specialists scientifically describe unpreventable ulcers and the causes, processing claims of negligence or malpractice raised against health agencies will be easier.
Differential Diagnosis
Evaluating tissue perfusion. When ruling out a diagnosis of bowel ischemia or lower extremity arterial occlusion or insufficiency, clear criteria are available to direct the work-up.38-41 However, direct observation is the only way to assess local skin and tissue perfusion. The NPUAP42 defines tissue perfusion in the absence of overt skin lesions or necrosis: “When compared to adjacent tissues or the opposite area of the body, the following changes are noted: skin temperature (coolness or increased warmth), firm or boggy texture, presence of pain or itching, persistent redness, or darkening of skin tone (red, bluish, or purple in dark-skinned people).”
In addition, capillary refill should be assessed every 8 hours for the first 24 hours. This is the key to differentiating between a Stage I pressure ulcer and deep tissue injury where the blood supply to the skin already has been obliterated by pressure necrosis. If the reddened skin area or lesion maintains capillary perfusion, the prognosis for recovery within the next 72 hours is good. No capillary refill – ie, no color change when fingertip pressure is applied – indicates poor prognosis and probable irreversible damage; the lesion will go on to demarcate and subsequently develop an eschar.13-17 The first 24 hours of admission are especially crucial; although capillary refill may appear to be present initially, the clinician actually may be observing a phenomenon known to forensic pathologists as hypostasis.43,44 This is a condition present in the early decomposition of dead tissue that mimics capillary refill. In actuality, it is “loose” blood, a product of capillary collapse. Hypostasis is rarely observed in critically ischemic tissues before complete occlusion – eg, rubor in ischemic lower extremities.45 Hypostasis can be manipulated for the first 8 to12 hours after tissue death but then becomes fixed (see Figure 2).43 In other words, dead tissues can mimic capillary refill. No capillary refill occurs in fixed hypostasis.
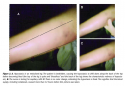 The best way to avoid mistaking early hypostasis for capillary refill is to examine the reddened area for capillary refill every 8 hours for the first 24 hours and document (see Figure 3). The findings of Nixon et al46 support this approach. Their preliminary study involving 31 elective surgery patients who developed a reddened area during a wide variety of different length procedures was conducted to diagnose early pressure ulcers using capillary refill and laser Doppler imaging. Capillary refill was recorded on all patients pre- and postoperatively (30 to 60 minutes after arrival in the Recovery Room and at 90 minutes) and daily for 8 days (or until discharge). The authors subdivided their definition of Stage I into Stage Ia and Ib (no blanching and blanching, respectively). No patients were classified as Stage Ib in the early postoperative period but eight converted to Stage Ib status on subsequent assessments. Laser Doppler images were drastically different. Stage Ia ulcers showed superficial increases in blood flow limited to the skin; Stage Ib exhibited increases in blood flow extending full-thickness to the bone with large areas of no blood flow.
The best way to avoid mistaking early hypostasis for capillary refill is to examine the reddened area for capillary refill every 8 hours for the first 24 hours and document (see Figure 3). The findings of Nixon et al46 support this approach. Their preliminary study involving 31 elective surgery patients who developed a reddened area during a wide variety of different length procedures was conducted to diagnose early pressure ulcers using capillary refill and laser Doppler imaging. Capillary refill was recorded on all patients pre- and postoperatively (30 to 60 minutes after arrival in the Recovery Room and at 90 minutes) and daily for 8 days (or until discharge). The authors subdivided their definition of Stage I into Stage Ia and Ib (no blanching and blanching, respectively). No patients were classified as Stage Ib in the early postoperative period but eight converted to Stage Ib status on subsequent assessments. Laser Doppler images were drastically different. Stage Ia ulcers showed superficial increases in blood flow limited to the skin; Stage Ib exhibited increases in blood flow extending full-thickness to the bone with large areas of no blood flow.
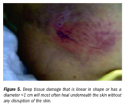
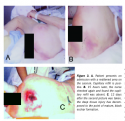 A deep tissue injury also can present as a pale, waxy-white area in light-skinned people or a lighter patch of skin surrounded by abnormally darker areas in dark-skinned people that shows no change in color when the capillary refill is tested. This presentation occurs when the individual has been resting on the area around the time the hypostasis is becoming fixed. The compression causes the loose blood to shift from the center to the edges of the dead tissue and become fixed in the peripheral dead tissues.47 This is most frequently seen in early sacral pressure damage (see Figure 4). A careful history of the individual’s mobility status and events before arrival at the healthcare facility often will reveal prolonged periods of immobilization and conditions that contribute to hypotension, hypercoagulability48 (eg, dehydration, cardiac decompensation, respiratory failure, infection/sepsis), and/or shock (example, hemorrhage, sepsis, spinal cord injuries). Deep tissue necrosis can occur and heal without eschar formation or with little disruption of the skin. These areas are small or narrow, having one diameter (length or width) <1 cm, and usually occur in fleshy areas as opposed to directly over bone. In these cases, enough collateral circulation is usually present and the area is small enough to heal rapidly (see Figure 5), although discoloration may take months to disappear.
A deep tissue injury also can present as a pale, waxy-white area in light-skinned people or a lighter patch of skin surrounded by abnormally darker areas in dark-skinned people that shows no change in color when the capillary refill is tested. This presentation occurs when the individual has been resting on the area around the time the hypostasis is becoming fixed. The compression causes the loose blood to shift from the center to the edges of the dead tissue and become fixed in the peripheral dead tissues.47 This is most frequently seen in early sacral pressure damage (see Figure 4). A careful history of the individual’s mobility status and events before arrival at the healthcare facility often will reveal prolonged periods of immobilization and conditions that contribute to hypotension, hypercoagulability48 (eg, dehydration, cardiac decompensation, respiratory failure, infection/sepsis), and/or shock (example, hemorrhage, sepsis, spinal cord injuries). Deep tissue necrosis can occur and heal without eschar formation or with little disruption of the skin. These areas are small or narrow, having one diameter (length or width) <1 cm, and usually occur in fleshy areas as opposed to directly over bone. In these cases, enough collateral circulation is usually present and the area is small enough to heal rapidly (see Figure 5), although discoloration may take months to disappear.
Contributions of forensic science. The explosion of forensic science over the last 15 years has been a boon to many areas of nursing and medical practice – eg, the processing and typing of DNA for “fingerprinting” criminals is now widely used to determine paternity/maternity. In his book Death’s Acre,49 forensic anthropologist Bill Bass describes the hour-by-hour, day-by-day post mortem changes that occur in bodies and their tissues.
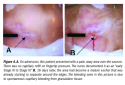
Dead tissue is assumed to behave the same way on living and deceased persons. The time line is longer on a living person because of 1) insect activity on corpses left out in the open (these cases are the best cases to review when researching changes in dead skin), 2) the presence of clothing and other coverings over the pressure injury that delays decomposition, and 3) the rapid decomposition of a corpse due to massive amounts of enzymes released by the dead tissue contribute to/expedite the process of autolysis. The term autolysis was first coined by forensic pathologists.47 On living persons, variables affecting tissue decomposition are fairly well controlled: body temperatures rarely fluctuate lower that 97˚ F or higher than 105˚ F and most of the time the body surface is covered either by clothing or bedclothes.50
Forensic studies also have revealed that moisture speeds up the decomposition of human tissues. Hence, drowning victims decompose much faster than victims lying out in the open air unless the water is very cold.51 If the air is very dry, even if it is warm, the decedent will mummify, exhibiting little decomposition except for his internal organs. As an analogy, deep pressure ulcers have been found to take longer to develop an eschar but the eschar autodebrides much faster with moist applications.52
The 7-day principle. Additional input into the timeline identifying the 2-week progressive decomposition of deep tissue injuries comes from general surgery. General surgeons have been teaching for years that the seventh postoperative day after surgery on the gastro-intestinal tract is a critical milestone in the recovery of the patient.53-55 Should the patient develop peritoneal signs or other signs of infection on the seventh day, an immediate re-exploration of the abdomen is recommended. The reason for the urgency in this scenario is that a portion of the bowel dies and on the seventh day it demarcates if any inadvertent devascularization of the bowel occurred during the initial surgery. Devascularization can result from either pressure on the anastomosis from edema or the lack of collaterals to perfuse the bowel after certain vascular procedures or wide resections of tissue or organs. Because bowel does not have the tensile strength of skin, it starts to disintegrate at the time it demarcates. Blunt abdominal trauma resulting in mesenteric injuries also will cause bowel ischemia and necrosis. As in other postoperative cases, the patient will be watched closely over the ensuing 7-day period. Wisner et al56 describe: “Two patients had delays in diagnosis of longer than 2 days. One was a 2-year-old child involved in a motor vehicle accident. Results of an abdominal computed tomographic scan performed shortly after admission were negative. The patient required exploration on the seventh day after injury because of abdominal tenderness and distention. A perforation of the distal ileum with peritonitis was discovered, and that segment of the ileum was resected….” Current improved techniques of peritoneal lavage in the emergency room result in diagnosing most of these injuries within minutes of arrival.57
This information is valuable because surgeons can pinpoint exactly when the interruption in perfusion occurred. For example, in the postoperative recovery phase, surgeons count forward from the day of the operation. For the purposes of deep pressure injuries that present as a demarcated red/purple area, clinicians can count back 7 days to pinpoint when the actual pressure damage occurred (see Figure 6).  The principle of the 7-day appearance of demarcation for devascularized tissues is substantiated by animal studies conducted by Harder et al58 and Junila et al,59 the former using myocutaneous flaps and the latter using devascularized skin. Harder also cites studies done on devascularized brain tissue.60,61 It appears that a wide variety of devascularized tissue types (and in many animal species) exhibit visible demarcation at 7 days without variability; the time taken for hypostasis to become fixed is variable.
The principle of the 7-day appearance of demarcation for devascularized tissues is substantiated by animal studies conducted by Harder et al58 and Junila et al,59 the former using myocutaneous flaps and the latter using devascularized skin. Harder also cites studies done on devascularized brain tissue.60,61 It appears that a wide variety of devascularized tissue types (and in many animal species) exhibit visible demarcation at 7 days without variability; the time taken for hypostasis to become fixed is variable.
Decomposition. Another interesting observation made by forensic pathologists is that the decomposition of tissues on the extremities is different than on the trunk. Although hypostasis and demarcation develop similarly in both anatomical locations, fluid-filled blisters and sloughs (“skin slippage”) are noted on the upper and lower extremities within a week if the body has not been refrigerated.47,62 Blistering is also frequently seen early on with deep pressure injuries of the lower extremities and sacrum after the area has demarcated and developed a deep purple to black discoloration (see Figure 7). In the case of blistering in a Stage II lesion, the wound bed remains pink and moist. In Stage III to Stage IV deep tissue injuries, the wound beneath the blister stays dark purple/black and when the skin sloughs, the wound bed is dry.
In summary, deep pressure injuries take 7 days from the early signs of redness to demarcate; by day 9 to 11 spontaneous “skin slippage” occurs and 14 to 15 days are required to form a mature brown/black eschar. This information is supported by observations made regarding devascularized skin from frostbite. The descriptions of eschar formation in frostbite mimic observations and data collected on eschars that form in deep tissue injury. Although the mechanism of injury is different (freezing of the tissues), the ultimate cause of tissue necrosis is ischemia from constricted capillaries and subsequent coagulation of the blood from stasis. Frostbite does not distort tissue configuration. The time of injury usually can be pinpointed within hours of occurrence; once the victim is restored to normal body temperature, the rate of dead tissue decomposition is the same as deep tissue injuries. Observations of frostbite reveal initial redness that lacks capillary refill, demarcation after 7 days, and skin slippage before eschar formation.63,64
As documentation of initial hypostasis fixation is compiled – ie, when the early absence of capillary refill is established – the exact timeline can be pinpointed with improved accuracy. For example, if a reddened area without capillary refill demarcates within 2 days of admission, it can be determined that the tissue died several days before admission.
Discussion
Understanding the dynamics that contribute to deep tissue pressure ulcers may be a factor in increasing their preventability and lowering the morbidity in a large segment of the sick and disabled. In a review of the literature,43 medical examiners and forensic scientists have determined that explicit, overt symptoms of tissue death start as early as 60 minutes from the time circulation ceases. In a decedent, circulation ceases when the heart stops; in a local block of tissue on a living person, circulation stops when the capillaries are no longer open or receiving inflow, such as when the larger pedicle has been sheared and kinked.
Frequent position changes help prevent prolonged ischemia of bony prominences from pressure and shearing. The current recommendation for position changes is every 2 hours.65 This turning schedule not only manages pressure, but also prevents the skin from overheating against the supporting surface.66 However, this standard is realistic only if pressure-relief surfaces are in place and positioning techniques that effectively distribute weight over a wide body surface are used. This first-line pressure management is so critical in preventing pressure ulcers given the acuity levels encountered in healthcare facilities today.
Limitations
Forensic science has been a great investigative force for determining the sources of many types of injuries as well as for providing autopsy verification of cause of death in many medical cases. However, with regard to pressure ulcers, forensic science has limitations. Although it can reveal the mechanism of injury surrounding a knife or gunshot wound on a living person, forensic science cannot be used to reconstruct events in wounds that have commenced to healing. Once an eschar has formed on a deep tissue injury, a myriad of internal and external conditions determine if and when the wound will autodebride or heal. If a pressure ulcer is debrided and in the stages of healing, accurately determining its age is difficult but ongoing in vivo human research is being conducted by the forensic community (eg, tissue assays) that may open the door to these sorts of determinations.
Implications for Practice and Research
Information extracted from research (including forensic scientists and pathologists) as well as expert observations and publications in nursing, surgery, and vascular disciplines can create a visible time frame against which other wound care clinicians can compare and record their observations and descriptions of deep tissue injuries. The information reviewed suggests that this theoretical temporal/visible framework be used to study and document deep tissue injuries in an effort to validate it as a systematic tool for further investigation. The older a deep tissue injury is along this 2-week continuum, the more definitive its appearance. Animal research has been particularly supportive in characterizing these changes. The next step will be the accumulation of corroborative experiences, observations of other specialists in the field, and the study of phenomena that fall outside this proposed path. These may include the effects of heparin on the appearance of deep tissue injuries compared with the information presented here and the effects of various topical treatments on the appearance of early deep tissue injuries. Do enzymatic debridement agents affect the final formation of an eschar? Can the use of scanners help corroborate this timeline? Laser Doppler scans and the newer skin sonogram technology would add another dimension to studying the changes in deep tissue injuries as they age and form eschars. The possibilities for future insights are far-reaching.
Just as scientists and forensic detectives have become experts in estimating the time of death for prosecutory or exculpatory evidence in court, wound care specialists can estimate the approximate age of a deep tissue injury up until the time of eschar formation and potentially bring additional scientific accuracy and discipline to their documentation. This has long-range implications for standardizing pressure ulcer prevention and treatment protocols across the board, enabling bedside clinicians and delivering pressure ulcer management into a new era.
Conclusions
Clinicians must be prepared to accurately determine diagnoses; diagnosis is the cornerstone of the entire care process. Without a diagnosis, planning, treating, and evaluating are impossible. In order to make a diagnosis of a Stage I pressure ulcer, clinicians must be able to rule out deeper damage.
An accurate diagnosis also has profound legal implications for hospitals, nursing homes, home care agencies, and patients. By identifying and documenting the characteristics of deep tissue injuries, establishing an estimated age of the injury based on the proposed timeline, and performing a retrospective review of patient events surrounding the time the injury happened, wound specialists may develop a reliable tool to research issues concerning pressure ulcer formation.
1. Black JM. Moving toward consensus on deep tissue injury and pressure ulcer staging. Adv Skin Wound Care. 2005;18(8):415-421.
2. Salcido R, Fisher SB, Donofrio JC, et al. An animal model and computer-controlled surface pressure delivery system for the production of pressure ulcers. J Rehabil Res Develop. 1995;32(2):149-161.
3. Bliss MR. Hyperaemia. J Tissue Viabil. 1998;8(4):4-13.
4. The difference between friction and shear. Adv Skin Wound Care. 2004;17(5):222.
5. Ankrom M, Bennett RG, Sprigle S, et al. Pressure-related deep tissue injury under intact skin and the current pressure ulcer staging systems. Adv Skin Wound Care. 2005;18(1):35-42.
6. National Database for Nursing Quality Improvement. NPUAP conference report. Nurs Qual News. 2005;6(1):1-2. Available at: www.nursingquality.org. Accessed September 17, 2005.
7. Shea JD. Pressure sores classification and management. Clin Orthoped. 1975;112:89-100.
8. Agency for Health Care Policy and Research Panel on the Prediction and Prevention of Pressure Ulcers in Adults. Clinical Practice Guideline No. 3, AHCPR publication No. 92-0047, Rockville, Md: May 1992.
9. Stage 1 ulcer definition revised for pigmentation: NPUAP now seeking endorsements. Wound Care News. 1998;4:40.
10. Bethel E. Controversies in classifying and assessing grade 1 pressure ulcers. Nurs Times. 2003;99(113):73-75.
11. Pires M, Muller A. Detection and management of early tissue pressure indicators: a pictorial essay. Progressions. 1991;3(3):3-11.
12. 2004 International Pressure Ulcer Prevalence Survey, Survey No. 7610, Marketing and Research Department. Hill Rom Services, Inc. Batesville, Ind.
13. Chauvapun JR, Dryjski M. Distal peripheral microembolism. Vascular. 2005;13(1):50-57.
14. Mayo RR, Swartz RD. Redefining the incidence of clinically detectable atheroembolism. Am J Med. 1996;100(5):524-529.
15. Blaisdell FW, Steele M, Allen RE. Management of acute lower extremity arterial ischemia due to embolism and thrombosis. Surgery. 1978;84(6):822-834.
16. Baumann DS, McGraw D, Rubin B. An institutional experience with arterial atheroembolism. Ann Vasc Surg. 1994;8(3):258-265.
17. Chakrabarty A, Phillips TJ. What is the diagnosis? Critical leg ischemia. WOUNDS. 2003;15(5):167-172.
18. Dimond B. Standard setting and litigation. Br J Nurs. 1994;3(5):235-238.
19. Dimond B. Pressure ulcers and litigation. Nurs Times. 2003;99(5):61-63.
20. Knowlton SP. The medical record: treatment tool or litigation device? Adv Skin Wound Care. 2003;16(2):97-98.
21. Johnson CE, Dobalian A, Burkhard J, Hedgecock DK, Harman J. Factors predicting lawsuits against nursing homes in Florida 1997-2001. Gerontologist. 2004;44(3):339-347.
22. Johnson CE, Dobalian A, Burkhard J, Hedgecock DK, Harman J. Predicting lawsuits against nursing homes in the United States 1997-2001. Health Serv Res. 2004;39(6 Pt 1):1713-1731.
23. What drives pressure ulcer classification – scientific knowledge or fear of litigation? J Tissue Viabil. 2005;15(2):2,4.
24. Voss AC, Bender SA, Ferguson ML, et al. Long-term care liability for pressure sores. J Am Geriatr Soc. 2005;53(9):1627-1629.
25. Bennett RG, O’Sullivan J, DeVito EM, Remsburg R. The increasing medical malpractice risk related to pressure ulcers in the United States. J Am Geriatr Soc. 2000;48(1):73-81.
26. Salcido R, Donofrio JC, Fisher SB, et al. Histopathology of pressure ulcers as a result of sequential computer-controlled pressure sessions in a fuzzy rat model. Adv Wound Care. 1994;7(5):23-24,26,28.
27. Nola GT, Vistnes LM. Differential response of skin and muscle in the experimental production of pressure sores. Plast Reconstruct Surg. 1980;66(5):728-733.
28. Aturaliya S, Lukasewycz A. Experimental forensic and bioanthropological aspects of soft tissue taphonomy: 1. Factors influencing post mortem tissue desiccation rate. J Forensic Science. 1999;44(5):893-896.
29. Daniel RK, Priest DL, Wheatley DC. Etiologic factors in pressure sores: an experimental model. Arch Phys Med Rehabil. 1981;62(10):492-498.
30. Tsuji S, Ichioka S, Sekiiya N, Nakatsuka T. Analysis of ischemia-reperfusion injury in a microcirculatory model of pressure ulcers. Wound Rep Regen. 2005;13(2):209-215.
31. Peirce SM, Skalak TC, Rodeheaver GT. Ischemia-reperfusion injury in chronic pressure ulcer formation: a skin model in the rat. Wound Rep Regen. 2000;8(1):68-76.
32. Stadler I, Zhang RY, Oskoui P, Whittaker MS, Lanzafame RJ. Development of a simple, noninvasive, clinically relevant model of pressure ulcers in the mouse. J Invest Surg. 2004;17(4):221-227.
33. Sibbald WJ. Update on current treatment modalities in shock. Available at: http://www.medscape.com/view article/420361. Accessed September 21, 2004.
34. Murphy RN. Legal and practical impact of clinical practice guidelines on nursing and medical practice. Adv Skin Wound Care. 1996;9(5):31-34.
35. Warner D. An expert’s perspective of the medical/legal chart review. ECPN. 2001;2:10-11.
36. Zukowski K, Langeno D, Posthauer ME, and the National Pressure Ulcer Advisory Panel. Coming to a consensus on deep tissue injury. Adv Skin Wound Care. 2005;18(1):28-29.
37. Whittington KT, Briones R. National prevalence and incidence study: 6-year sequential acute care data. Adv Skin Wound Care. 2004;17(9):490-494.
38. Greenfield LJ, Mulholland MW, Oldham KJ, Zelenock GB, Lillemoe KD. Surgery: Scientific Principles and Practice, 3rd ed. Philadelphia, Pa: Lippincott Williams and Wilkins;2001:1818-1819.
39. Tollefson DFJ, Ernst CB. Colon ischemia following aortic reconstruction. In: Porter JM, Taylor LM jr, eds. Basic Data Underlying Clinical Decision Making in Vascular Surgery. St. Louis, Mo: Quality Medical;1994.
40. Rutherford RB. Vascular Surgery, 5th ed. Philadelphia, Pa: WB Saunders Co;2000;813-821.
41. Geraghty PJ, Sanchez LA, Rubin BG, et al. Overt ischemic colitis after endovascular repair of aortolliac aneurysms. J Vasc Surg. 2004;40(3):413-418.
42. National Pressure Ulcer Advisory Panel. Stage 1 assessment in darkly pigmented skin. 1998, Reston, Va. Available at: http://www.mpuap.org/positn4.html. Accessed December 10, 2004.
43. Sauko P, Knight B. Knight’s Forensic Pathology, 3rd ed. London, UK: Arnold Press;2004.
44. Henssge C, Knight B, Krompecher T, Madea B, Nokes L. The Estimation of the Time of Death in the Early Postmortem Period. Boston, Mass: Edward Arnold;1995:219.
45. Seiggreen MY, Kline RA. Arterial insufficiency and ulceration: diagnosis and treatment options. Adv Skin Wound Care. 2004;17(5 Pt 1):242-251.
46. Nixon J, Smye S, Scott J, Bond S. The diagnosis of early pressure ulcers: report of the pilot study. J Tissue Viabil. 1999;9(2):62-66.
47. Dix J, Graham M. Time of Death, Decomposition and Identification. New York, NY: CRC Press;2000:16-19.
48. Shankar VK, Chaudhury SR, Uthappa MC, Handa A, Hands LJ. Changes in blood coagulability as it traverses the ischemic limb. J Vasc Surg. 2004;39(5):1033-1042.
49. Bass W, Jefferson J. Death’s Acre. New York, NY: The Penguin Group, Inc;2004:10-11.
50. Mall G, Eckl M, Sinicina I, Peschel O, Hubig M. Temperature-based death time estimation with only partially known environmental conditions. Int J Legal Med. 2005;119(4):185-194.
51. Haglund WD. Disappearance of soft tissue and the disarticulation of human remains from aqueous environments. J Forensic Sci. 1993;38(4):806-815.
52. van Rijswijk L. Moist dressings: bridging the gap between research and practice. Adv Skin Wound Care. 2004;17(5 Pt 1):254-255.
53. Mattox KL, Feliciano DV, Moore EE. Trauma. New York, NY: McGraw-Hill;2000:720.
54. Schwartz SI, Shires GT, Spencer FC, Husser WC. Principles of Surgery, 6th ed. New York, NY: McGraw-Hill, Inc;1994:457.
55. Sabiston DC. Textbook of Surgery: The Biological Basis of Modern Surgical Practice, 13th ed. Philadelphia, Pa: WB Saunders Company;1986.
56. Wisner DH, Chun Y, Blaisdell FW. Blunt intestinal injury. Arch Surg. 1990;125(10):1319-1323.
57. Dauterive AH, Flancbaum L, Cox EF. Blunt intestinal trauma. A modern-day review. Ann Surg. 1985;201(2):198-203.
58. Harder Y, Amon M, Georgi M, Banic A, Erni D, Menger MD. Evolution of a “falx lunatica” in demarcation of critically ischemic myocutaneous tissue. Am J Physiol Heart: Circulation and Physiology. 2005;288(3):H1224-H1232.
59. Junila J, Kaarela O, Waris T. The formation of the demarcation line at experimental frostbite. Int J Circumpolar Health. 1999;58(1):44-51.
60. Astrup J, Siesjo BK, Symon L. Thresholds of cerebral ischemia – the ischemic penumbra. Stroke. 1981;12(6):723-725.
61. Astrup J, Symon L, Branston N, Tassen N. Thresholds of cerebral ischemia. In: Schmiedek P (ed). Microsurgery for Stroke. Berlin, Germany: Springer;1976:16-21.
62. DiMaio DJ, DiMaio VJ. Forensic Pathology. New York, NY: CRC Press;1993:24-28.
63. Lehmuskallio E, Lindholm H, Koskenvuo K, Sarna S, Friberg D, Viljanen A. Frostbite of the face and ears: epidemiological study of risk factors in Finnish conscripts. Br Med J. 1995;311(7021):1661-1663.
64. Lipton L. Understanding frostbite’s bite. Available at: www.merginet.com/index.cfm?pg=environ&fn=frostbite.
65. Salcido R. Patient turning schedules: why and how often. Adv Skin Wound Care. 2004;17(4 Pt 1):156.
66. Lachenbruch C. Skin cooling surfaces: estimating the importance of limiting skin temperature. Ostomy Wound Manage. 2005;51(2):70-79.













