Sound Evidence: Acoustic Pressure Wound Therapy for Management of Mixed Partial- and Full-Thickness Burns in a Rural Wound Center
More than one million burn injuries occur in the US annually, resulting in more than 500,000 emergency department visits and 40,000 hospitalizations.1 While most burns can be treated on an outpatient basis,2 treatment can be difficult without specialized care.3,4
Burns are classified by depth of tissue damage. Partial-thickness burns can extend to the dermis and are usually extremely painful. Healing can occur within 10 to 14 days, depending on wound size.5 Full-thickness burns extend to the subcutaneous layer and are typically painless due to nerve destruction. Surgery and skin grafts are usually required for full-thickness burns.6 Burn treatment varies according to type, depth, and extent of injury. Standard burn treatment includes daily cleansing, debridement, application of topical antimicrobials, nonadherent dressings, and pain management.4
Acoustic pressure wound therapy (APWT; MIST® Therapy System [Celleration, Eden Prairie, MN]), is a noncontact, low-frequency, nonthermal ultrasound treatment utilizing positive pressure to accelerate healing, cleanse, and debride. In APWT, ultrasound waves delivered via sterile saline mist stimulate fibroblasts to accelerate healing and remove bacteria from the wound bed.7-9 The effectiveness of APWT has been demonstrated in acute and chronic wounds,10-13 including burns,14 and has been associated with reductions in wound-related pain.15
This retrospective case series study was conducted to assess the clinical effectiveness of APWT in treating mixed partial- and full-thickness burns in a rural wound care center lacking specialized burn care.
Methods
Data were manually extracted from the charts of 14 consecutively treated outpatients with mixed partial- and full-thickness burns who received APWT with standard burn care from August 2006 to October 2007. Data included demographic characteristics (age, sex, and ethnicity), medical histories, burn data (date of burn, type, severity, dimensions, and percent body surface area affected), total number of APWT treatments necessary for complete healing, pre- and post treatment pain scores, and the number of APWT treatments necessary to decrease pain. Burn thickness was assessed by clinical appearance. Burns were classified as full-thickness if eschar was present and no blanching was evident with pressure. All burns had areas of partial-thickness. Treatment continued until burns were healed. Patients were followed for up to 6 months.
Outcome effectiveness of APWT was evaluated based on scarring characteristics (ie, cosmetic appearance) of healed wounds and pain resolution. The authors determined scarring characteristics by visual inspection. Patients rated pain using a 10-point visual analog scale (VAS; 0 = no pain, 10 = severe pain) before each APWT treatment.
Summary of Cases
Patients were 5 months to 78 years old.  Seven patients (50%) had medical histories significant for diabetes or cardiovascular disease (see Table 1). Most (13 out of 14) burns were thermal; one was chemical. Burns were located on extremities, trunk, or both. The average body surface area affected was 7% (range: 1% to 24%). Most patients (71%) experienced pre-treatment burn-related pain (VAS 2 to 10).
Seven patients (50%) had medical histories significant for diabetes or cardiovascular disease (see Table 1). Most (13 out of 14) burns were thermal; one was chemical. Burns were located on extremities, trunk, or both. The average body surface area affected was 7% (range: 1% to 24%). Most patients (71%) experienced pre-treatment burn-related pain (VAS 2 to 10).
Patients received between two and 108 APWT treatments, depending on their rate of healing (see Table 1). Topical treatments included antimicrobials (eg, silver sulfadiazine 1% and hydrofiber with silver) and petroleum gauze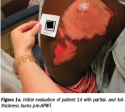 dressings; one patient received enzymatic debridement and whirlpool treatments (pre-APWT).
dressings; one patient received enzymatic debridement and whirlpool treatments (pre-APWT).
Pain resolved within one and 10 APWT treatments. No hospitalizations or burn infections occurred. Pliable, nonhypertrophic (ie, flat) scars developed in 86% of patients (see Figure 1a-c). Repigmentation developed in 79% of patients, with cosmetically acceptable appearance in 71%. All burns healed between 1 week and 45 weeks.
Follow-up data were available for 71% of patients; nine at 6 months and one at 3.5 months (this patient died of unrelated causes before the 6-month follow-up). Initial scar assessments 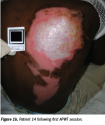 (see Table 1) were unchanged at follow-up. Four patients were lost to follow-up.
(see Table 1) were unchanged at follow-up. Four patients were lost to follow-up.
Conclusion
The effectiveness of APWT in outpatient care for mixed partial- and full-thickness burns was demonstrated in 14 patients through cosmetically acceptable scarring (ie, predominantly pliable, nonhypertrophic scars and repigmentation) and pain reduction. No patient developed infectious complications. Initial scar assessments were maintained through follow-up. As this was not intended as a comparative trial, no comparison to treatment without APWT is possible nor is literature available as a historic control in a similar population.
The mixed partial- and 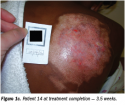 full-thickness burns in this retrospective study healed without surgery or skin grafts typical for serious and extensive burns.2 Based on the results of this limited case series, studies of APWT as an adjunct to standard therapies for inpatient burn management are warranted.
full-thickness burns in this retrospective study healed without surgery or skin grafts typical for serious and extensive burns.2 Based on the results of this limited case series, studies of APWT as an adjunct to standard therapies for inpatient burn management are warranted.
Acknowledgment
Tracey Fine, MS, ELS, assisted with manuscript preparation.
1. American Burn Association. Burn Incidence and Treatment in the US: 2007 Fact Sheet. Chicago: American Burn Association;2007. Available at www.ameriburn.org/resources_factsheet.php. Accessed February 22, 2008.
2. Edlich RF, Drake DB, Long WB. Burns, thermal. eMedicine 2007. Available at www.emedicine.com/plastic/topic518.htm. Accessed January 3, 2008.
3. Sagraves SG, Phade SV, Spain T. A collaborative systems approach to rural burn care. J Burn Care Res. 2007;28(1):111-114.
4. Moss L. Outpatient management of the burn patient. Crit Care Nurs Clin N Am. 2004;16(1):109-117.
5. McCain D, Sutherland S. Nursing essentials: skin grafts for patient with burns. Am J Nurs. 1998;98(7):34-39.
6. Myers B. Wound Management Principles and Practice. 1st ed. Upper Saddle River, NJ: Prentice Hall Co;2004.
7. Lai JY, Pittelkow MR. Physiological effect of ultrasound mist on fibroblasts. Int J Dermatol. 2007;46(6):587-593.
8. Houghton PE, Thawer HA. Effects of ultrasound delivered through a mist of saline to wounds in mice with diabetes mellitus. J Wound Care. 2004;13(5):1-6.
9. Wagner SA, Kavros SJ, Vetter EA, Cockerill FR. The effect of MIST ultra-sound transport technology on common bacterial wound pathogens. Presented at 14th annual Symposium on Advanced Wound Care, Las Vegas, Nevada. May 2, 2001.
10. Mohr P, Stegmann W, Breitbart EW. Low-frequency ultrasound treatment of chronic venous ulcers. Wound Repair Regen. 1997;5:18-22.
11. Ennis WJ, Vadles W, Gainer M, Meneses P. Evaluation of clinical effectiveness of MIST ultrasound therapy for the treatment for the healing of chronic wounds. Adv Skin Wound Care. 2006;19:437-446.
12. Ennis WJ, Formann P, Mozen N, et al. Ultrasound therapy for recalcitrant diabetic foot ulcers: results of a randomized, double-blind, controlled, multicenter study. Ostomy Wound Manage. 2005;51(8):24-39.
13. Kavros SJ, Miller JL, Hanna SW. Treatment of ischemic wounds with noncontact, low-frequency ultrasound: the Mayo clinic experience, 2004-2006. Adv Skin Wound Care. 2007;20(4):221-226.
14. Haan J, Lucich S. MIST Therapy® System – thoughts on therapy: case series #2. ECPN. 2007;116(2):39-43.
15. Gehling ML, Samies JH. The effect of noncontact, low-intensity, low-frequency therapeutic ultrasound on lower-extremity chronic wound pain: a retrospective chart review. Ostomy Wound Manage. 2007;53(3):44-50.













