Acellular Bovine-Derived Matrix Used on a Traumatic Crush Injury of the Hand: A Case Study
Abstract
Wound care options that provide quality care at a reasonable cost are integral to limb salvage and everyday treatment approaches in patients with traumatic injuries and nonhealing, chronic wounds. Human skin substitutes, or bioengineered tissues, have been available for many years but data about the use, safety, and effectiveness of bovine-derived bioengineered products are limited. A 50-year old man was seen at the wound clinic with a failing skin graft 65 days after sustaining a crush injury of his dominant hand.
Following the injury, the patient underwent amputation of the ring and little finger, a revascularization procedure, and an open reduction and internal fixation of the right thumb and long finger. Following debridement, two applications of bovine-derived bioengineered tissue resulted in wound closure, limb preservation, and maximum functional use. Use of hydrogel-soaked and petrolatum-impregnated gauze as secondary and tertiary dressings, respectively, was needed to help maintain optimal bioengineered tissue moisture levels. Additional clinical studies to assess the use, safety, and cost effectiveness of this treatment modality are warranted.
Please address correspondence to: Amy P. Dunckel, PT, DPT, St. John’s Therapy Services-Lebanon, 331 Hospital Drive, Lebanon, MO 65336; email: amyptschool@hotmail.com.
In the face of changing healthcare economics, the need to provide quality care at reduced costs is increasing.1 The cost of healing chronic and nonresponsive ulcers can be substantial. For years, surgical intervention has been considered one of the most viable options for chronic and nonhealing wounds. Even today, surgical intervention, including autologous skin grafting, is still considered one of the gold standards in the treatment of traumatic wounds and various types of nonhealing ulcers.2-4 As medical advances improve the survival rates for patients with catastrophic injuries, it is becoming more apparent that alternative methods of treatment must be explored; often, large surface areas require coverage, yet available donor tissue is limited.5 This gold standard has undeniable disadvantages, including the inherent risks of surgical procedures, potential risk of increased pain to the patient, increased integument interruption, increased risk of infection, and possible fluid and electrolyte imbalance created by the donor site.2 As a result, human skin substitutes have been the focus of many clinical trials in an attempt to address wounds that require healing by secondary intention.5 Many of these clinical trials are outlined by Shores et al.6
Due to their compact size and functional maneuverability, the fingers and hands are particularly vulnerable to mutilating injury.7 In 2001, an estimated 4.8 million patients were treated in emergency departments across the US for nonwork-related hand and finger injuries.7 Sorock et al’s8 review of the literature showed that in the mid 1990s cuts and lacerations to the hand were the third leading cause of lost workday cases in the US. In 2000, the National Institute for Occupational Safety and Health (NIOSH)9 reported that 94% of occupational-related crush and laceration amputations involved the fingers. The  psychological impact of these injuries goes far beyond the accidents — often, patients suffering catastrophic and disfiguring injuries develop anxiety reactions that include some level of post-traumatic stress disorder, depressive symptoms, and grief and body image disturbances, as well as complaints of chronic pain.9 As such, physicians and surgeons know that preserving vital structures to minimize the degree of cosmetic disfigurement while maintaining acceptable function leaves little room for error.10
psychological impact of these injuries goes far beyond the accidents — often, patients suffering catastrophic and disfiguring injuries develop anxiety reactions that include some level of post-traumatic stress disorder, depressive symptoms, and grief and body image disturbances, as well as complaints of chronic pain.9 As such, physicians and surgeons know that preserving vital structures to minimize the degree of cosmetic disfigurement while maintaining acceptable function leaves little room for error.10
The distinctive structure and function of the hand make it a uniquely challenging site for the wound care therapist.11 Any effective method to deal with tissue loss in this area must result in a healed wound that is strong enough to maintain consistency yet soft and supple enough to conform to the various movements afforded the remaining portion of the hand. Bioengineered tissues, especially porcine derived xenografts, have been available and successfully used for many years. Data about the use, safety, and effectiveness of bovine-derived tissue are limited. The purpose of this case study is to describe the use of bovine-derived tissue in the treatment of a complex traumatic crush injury of the hand treated by the wound care therapist.
Case Report
History. Mr. Y was an otherwise healthy 50-year-old waste industries manager who presented to the clinic following surgical repair of a complex crush injury/partial amputation of the right hand, subsequent to an accident involving the machinery blades of a garbage truck. According to his patient record and detailed operative notes, immediately following the initial injury Mr. Y had been taken to the operating room for extensive debridement of the hand and amputation of the ring and little fingers. In addition, Mr. Y had angiographic evidence of devascularization of the superficial palmar arch with subsequent critical limb ischemia to the thumb, middle finger, ring finger, and little finger, requiring revascularization. Also, an open reduction, internal fixation of the right thumb and long finger were performed. The day after these procedures, he returned to the operating room for additional debridement of nonviable tissue and allograft placement. However, 49 days later, with poor wound healing and an unsuccessful pinning, Mr. Y returned to the operating room for hardware removal, a revised fixation of the third metacarpal fracture, and a split-thickness skin graft to cover the nonhealing wound and what remained of the ulnar border of his hand.
Following this surgical procedure, Mr. Y received home health care to perform his dressing changes (he was not specific on types or frequency of dressing changes) and had been seen by the resident occupational therapist for fabrication of a resting night splint that he continued to wear every night. No other form of therapy had been instituted. Mr. Y arrived at the author’s clinic 15 days following his most recent procedure and 65 days post injury (see Figure 1) for his initial physical therapy examination.
 Management. Although wrist and hand range-of-motion measurements were obtained during Mr. Y’s initial evaluation, as well as bimonthly progress notes, the primary focus of the initial evaluation performed at the author’s facility concentrated on the wound itself, as well as the associated edema that remained. Mr. Y reported minimal to no current pain. Even though the thumb and forefinger were intact, functional limitations included severe loss of fine motor skills and complete functional loss of the middle finger because no extensor mechanism remained. Although Mr. Y reported he was able to perform all activities of daily living independently, he experienced difficulty with buttons and zippers, especially when wearing jeans. In addition, he had increased the use of his left upper extremity and hand in lieu of attempting many activities with his right hand. As a result of his injury, he was unable to return to normal work duties and was placed on disability leave.
Management. Although wrist and hand range-of-motion measurements were obtained during Mr. Y’s initial evaluation, as well as bimonthly progress notes, the primary focus of the initial evaluation performed at the author’s facility concentrated on the wound itself, as well as the associated edema that remained. Mr. Y reported minimal to no current pain. Even though the thumb and forefinger were intact, functional limitations included severe loss of fine motor skills and complete functional loss of the middle finger because no extensor mechanism remained. Although Mr. Y reported he was able to perform all activities of daily living independently, he experienced difficulty with buttons and zippers, especially when wearing jeans. In addition, he had increased the use of his left upper extremity and hand in lieu of attempting many activities with his right hand. As a result of his injury, he was unable to return to normal work duties and was placed on disability leave.
Wound measurements. Due to the size and location of this patient’s wound, simple linear measurements were chosen as the method of choice for wound bed measurements, a method of assessment proven both simple, cost efficient, and reliable.12,13 Initial evaluation showed the presence of a skin graft measuring 11.0 cm in length and 8.2 cm in width on the medial aspect of the hand. An open area at the distal portion of the graft, measured using simple clock reference principles,14 was 3.5 cm in length and 4.2 cm wide. Wound depth could not be determined due to the amount and level of nonviable tissue in the wound bed. Minimal sanguineous exudate (as evidenced when the bandage was removed) and no odor were noted. Before debridement, the wound contained approximately 30% yellow slough, 55% brown and black necrotic tissue, and 15% healthy granulation tissue. The entire periwound was blackened with what appeared to be dried blood and the graft appeared very fragile.
Girth measurements were obtained to assess edema; wound healing difficulties were expected due to the presence of post-traumatic and postsurgical fluid in the tissues. Of the two primary methods for determining the amount of edema in the limb of a patient — tape measure and water displacement (volumetric) measurements15,16 — the tape measure method was deemed the most appropriate for Mr. Y because he had an open wound. Girth measurements were taken at the thumb and the joints of the remaining index and middle fingers at every bimonthly evaluation (see Table 1). Contralateral results were compared at the initial evaluation only. Although girth measurements were repeated multiple times throughout the course of this patient’s care, the data located in the chart represent initial evaluation measurements, approximate mid-point measurements, and measurements taken at discharge.
 Wound cleansing and dressing. At the time of his initial evaluation, Mr. Y was scheduled for clinic visits every Monday, Wednesday, and Friday. The wound was cleansed with normal saline followed by blunt debridement with tweezers and scissors to remove necrotic and nonresponding portions of the graft while preventing disruption of the areas that remained intact. The wound was dressed with petrolatum-impregnated gauze to help soften the remaining nonviable tissue and allow for easy removal; this was covered with an absorbent dressing (dry gauze) to collect any wound drainage.
Wound cleansing and dressing. At the time of his initial evaluation, Mr. Y was scheduled for clinic visits every Monday, Wednesday, and Friday. The wound was cleansed with normal saline followed by blunt debridement with tweezers and scissors to remove necrotic and nonresponding portions of the graft while preventing disruption of the areas that remained intact. The wound was dressed with petrolatum-impregnated gauze to help soften the remaining nonviable tissue and allow for easy removal; this was covered with an absorbent dressing (dry gauze) to collect any wound drainage.
Prognosis. For the next 3 weeks, Mr. Y was seen in the clinic and most nonviable tissue was removed successfully. However, the ulnar border of his hand remained unhealed. Mr. Y returned to the operating room for an additional split-thickness skin graft. To aid in recovery, all active and passive range-of-motion exercises of the remaining fingers were discontinued and care was taken with wrist range-of-motion exercises to prevent disruption of the surgical site. Despite cooperation with this care regimen, Mr. Y’s graft began to demonstrate signs of nonadherence within 6 days and was removed 2 days later.
Changing the Approach
One hundred days post initial injury and 36 days from initial evaluation, one small nonhealing wound continued to jeopardize limb preservation. At this time, Mr. Y’s physician agreed to try a prescription bioengineered tissue substitute. Following consultation with the trauma unit at the author’s sister facility, the recommendation was made to use an acellular dermal matrix dressing, Primatrix™ (TEI Biosciences, Boston MA). This FDA-approved dressing consists of a fetal bovine-derived dermal scaffold and is designed for use in a variety of wounds, including traumatic wounds.
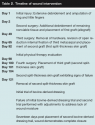 On day 101, Mr. Y was seen in the clinic and agreed to try the bovine-derived dressing to help heal the remaining wound that measured 1.7 cm x 3.2 cm and was 0.3 cm at its deepest point (located at approximately 3 o’clock). The wound bed contained 10% slough coverage and 90% healthy granulation tissue and wound drainage was minimal.
On day 101, Mr. Y was seen in the clinic and agreed to try the bovine-derived dressing to help heal the remaining wound that measured 1.7 cm x 3.2 cm and was 0.3 cm at its deepest point (located at approximately 3 o’clock). The wound bed contained 10% slough coverage and 90% healthy granulation tissue and wound drainage was minimal.
The wound was cleansed with normal saline and all remaining nonviable tissue was debrided with tweezers and scissors until a clean wound edge was apparent. Per the manufacturer guidelines,17 a sterile piece of the bovine-derived dressing was cut to fit just beyond the wound edges and hydrated utilizing room-temperature, sterile 0.9% saline until the dressing turned from white to light grey (appearing slightly translucent). The hydrated dressing was applied and held in place with 3M™ Steri-Strip™ Adhesive Skin Closures (St. Paul, MN) (see Figure 2).
The covered wound was dressed with a petrolatum-impregnated gauze to help maintain a moist wound environment, wrapped boxer’s glove style with a gauze roll, and held together with meshed tubing. Maintaining a moist wound 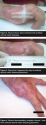 environment is vital to wound healing because it allows for autolytic debridement to occur, helps prevent infections, and facilitates healing.18
environment is vital to wound healing because it allows for autolytic debridement to occur, helps prevent infections, and facilitates healing.18
Following dressing placement, Mr. Y was instructed to visit the clinic daily to verify proper wound bed hydration (as evidenced by the grey and slightly translucent appearance of the dressing). Range-of-motion activities in the fingers and hand were discontinued. Within 3 days, it was apparent by the yellow color of the dressing that proper hydration had not been achieved with this protocol and the initial trial of the bovine-derived dressing ended with dressing removal. The wound had not changed in size.
A second trial of the bovine-derived dressing was initiated. The protocol was altered to include a hydrogel-soaked gauze dressing for increased moisture underneath the petrolatum-impregnated gauze. The wound was covered with additional dressings in the same manner as previously described. Mr. Y was seen three times per week for reapplication of hydrating dressings.
Seventeen days post placement of the bovine-derived dressing, the wound was fully re-epithelialized and was considered closed 2 weeks following the completion of wound healing.5 Figure 3 and Figure 4 illustrate Mr. Y’s progression from initial placement of the bovine-derived dressing to 1 year post-injury.
Discussion
Human skin is one of the most highly complex and frequently disrupted organs.18 The skin protects the body by regulating internal temperature, preventing dehydration, and providing an effective barrier against environmental pathogens.19 Methods to repair skin damage have been the topic of noteworthy study and research in the last several decades. Healthcare costs to manage acute and chronic wounds include direct costs such as caregiver time, hospitalization, medications, dressings, and supplies as well as indirect costs such as lost time and productivity at work, quality-of-life alterations, pain, depression, and possible infection.20,21 Although initial unit cost per bandage for specialty treatments, such as the use of bioengineered tissue, may be high, the overall cost of care may be decreased due to improved rates of wound healing.22
Recent case series and case study reports detail utilization of bioengineered tissues to treat a variety of wounds that include venous ulcers,23,24 diabetic wounds and pressure ulcers,25 and wounds related to recessive dystrophic epidermolysis bullosa26,27 and cancer excisions.5,24,28 When comparing bioengineered tissues and skin grafting procedures side by side, trials utilizing bioengineered tissue revealed they performed just as well as many skin grafting procedures and in some cases decreased healing times,24,28 decreased dressing change frequency,28 and allowed for symptom relief,26 all while demonstrating minimal to no toxic effects or product rejection.29,30 In addition, research in the field of tissue integrity5,6,23,28,29 reveals that recent advances in bioengineered tissue have not only achieved cutaneous repair, but also may provide improved aesthetic benefits while offering a readily available tissue alternative to autologous grafts. In vivo studies25 have shown that utilizing human skin equivalents aids in augmenting cells and growth factors that normally may be deficient in the nonhealing wound. Plus, although many of the published clinical trials performed utilizing xenografts for healing chronic and nonresponsive wounds involve porcine-derived dressings,6 the use of bovine-derived dermal matrix dressings is gaining interest. In a prospective, randomized study by Falanga et al,30 293 patients with venous insufficiency ulcers demonstrated no clinical signs of rejection and no evidence of post-healing tissue breakdown involving the use of bilayered human skin equivalents consisting of type I bovine collagen and containing living human dermal fibroblasts. Use of type I bovine-derived collagen following surgically created wounds has been shown in pilot studies28 to decrease the need for frequent dressing changes because collagen matrix products can safely remain in wounds for prolonged periods of time. In addition, this study also revealed that bovine-derived dressings have a low antigenic potential; therefore, they act as a poor medium for bacterial growth.28 Finally, a review of research19 performed during the last 50 years has shown that although the collagen base to most of the dermal matrix dressings has a mild immunoreactivity, only approximately 3% of the population will develop a hypersensitivity to an initial skin challenge of bovine collagen. Thus, it can be ascertained that bovine-derived dressings demonstrate a low immunologic response and a high level of safety for the majority of patients.2
Although Mr. Y demonstrated notable healing before placement of the bovine-derived dressing, the remaining open portion of his hand posed a threat to overall limb preservation. Following one failed cadaveric allograft and two failed split-thickness autografts, consideration of a bioengineered tissue substitute seemed appropriate. According to recent case studies31 and biological tissue research,28 the fetal bovine matrix scaffold aids in establishing a wound environment that supports cell repopulation and angiogenesis and integrates collagen into the bed of the wound. The results of this case study suggest that this substitute can be safely used in outpatient care settings and confirm that maintaining an appropriate wound environment is crucial to the survival of the tissue.
Conclusion
As options in wound care increase and the ability to keep patients alive following catastrophic injuries improves, the use of advanced tissue technologies enhances the role clinicians can play in positive patient outcomes. Recent advances in the field of bioengineered skin substitutes may allow for a greater number of patients to be considered appropriate candidates for reconstructive procedures to achieve wound closure and limb preservation.6
This case study demonstrated a successful outcome following the use of bioengineered tissue; however, consideration must be given to the fact that this patient was compliant with all wound care protocols and may have done well with one of the many other bioengineered tissue substitutes. This case suggests that the use of bovine-derived dermal matrix dressings may be an appropriate treatment option for wounds that have been nonresponsive to more conventional wound closure methods. However, as this is only one case, caution should be used in generalizing its effectiveness in the vast arena of wound care. Additional clinical trials of bovine-derived products, including studies to assess treatment safety and compare its efficacy and cost effectiveness to other available treatment options, are warranted.
Acknowledgment
The author thanks Samantha Marocco, PT, MS, GCS, and Leslie Russek, PT, DPT, PhD, OCS for their assistance with this manuscript.
1. Rees RS, Hirshburg JA. Wound care centers: costs, care and strategies. Adv Wound Care. 1999. Available at: www.findarticles.com/p/articles/mi_qa3964/is_199907/ai_n8859694/. Accessed September 28, 2009.
2. Zbigniew R, Schwartz RA. Modern aspects of wound healing: an update. Dermatol Surg. 2000;26:219–229.
3. Khachemoune A, Welsh R, Ehrsam E. An elderly woman with a nonhealing ulcer. Am Fam Phys. 2005;71:2163–2165.
4. Hohlfeld J, de Buys Roessingh A, et al. Tissue engineered fetal skin constructs for pediatric burns. Lancet. 2005;366:840–842.
5. Gohari S, Gambla C, Healey M, et al. Evaluation of tissue-engineered skin (human skin substitute) and secondary intention healing in the treatment of full thickness wounds after Mohs micrographic or excisional surgery. Dermatol Surg. 2002;28:1007–1114.
6. Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Care. 2007;20:493–508.
7. Conn M, Annest J, Ryan G, et al. Non-work-related finger amputations in the United States, 2001–2002. Ann Emer Med. 2005;45:630–635 .
8. Sorock G, Lombardi D, Courtney T, et al. Epidemiology of occupational acute traumatic hand injuries: a literature review. Safety Science. 2001;38:241–256.
9. Wald J, Alvaro R. Psychological factors in work-related amputation: considerations for rehabilitation counselors. J Rehabil. 2004;70:6–15.
10. del Pinal F. Severe mutilating injuries to the hand: guidelines for organizing the chaos. J Plast Reconstr Aesthet Surg. 2007;60:816–827.
11. Bueno R, Neumeister M. Outcomes after mutilating hand injuries: review of the literature and recommendations for assessment. Hand Clin. 2003;19:193–204.
12. Goldman R, Salcido R. More than one way to measure a wound: an overview of tools and techniques. Adv Skin Wound Care. 2002;15:236–243.
13. Majeske C. Reliability of wound surface area measurements. Phys Ther. 1992;72:57–60.
14. Scarborough-Roessler P. Clinical Debridement Skills. Presentation at the Educators 2000 Plus. Kansas City, MO. August 2, 2002.
15. Karges JR, Mark BE, Stikeleather SJ, et al. Concurrent validity of upper-extremity volume estimates: comparison of calculated volume derived from girth measurements and water displacement volume. Phys Ther. 2003;83:134–146.
16. Taylor R, Jayasinghe UW, Koelmeyer L, et al. Reliability and validity of arm volume measurements for assessment of lymphedema. Phys Ther. 2006;86:205–214.
17. Primatrix Clinical Application Guidelines. Available at: www.teibio.com/Literature/PriMatrix/. Accessed November 1, 2007.
18. Harvey C. Wound healing. Orthopaed Nurs. 2005;24:143–159.
19. Balasubramani M, Kumar T, Babu M. Skin substitutes: a review. Burns. 2001;27:534–544
20. Bolton LL, van Rijswijk L, Shaffer FA. Quality wound care equals cost-effective wound care. Nurs Manag. 1996;27:30–33.
21. Driver V. Health economics of wound care and limb preservation: beyond clinical evidence. Diabet Microvasc Complications Today. 2006;4:29–32.
22. Edwards M. The Informed Practice Nurse, 2nd ed. West Sussex, England: John Wiley & Sons Ltd;2008.
23. Singer A, Clark RAF. Cutaneous wound healing. N Eng J Med. 1999;341:738–746.
24. Muhart M, McFalls S, Kirsner RS, et al. Behavior of tissue-engineered skin. Arch Dermatol. 1999;135:913–918.
25. Brem H, Balledux J, Bloom T, et al. Healing of diabetic foot ulcers and pressure ulcers with human skin equivalent: a new paradigm in wound healing. Arch Surg. 2000;135:627–634.
26. Sibbald G, Zucker R, Coutts P, et al. Using a dermal skin substitute in the treatment of chronic wounds secondary to recessive dystrophic epidermolysis bullosa: a case series. Ostomy Wound Manage. 2005;51:22.
27. Witt P, Cohen DT, Mallory SB. Use of permanent acellular dermal allograft in recessive dystrophic epidermolysis bullosa involving hands. Arch Dermatol. 1999;135:503–506.
28. Kolenik SA, McGovern TW, Leffell DJ. Use of lyophilized bovine collagen matrix in postoperative wound healing. Dermatol Surg. 1999;25:303–307.
29. Badylak S. The extracellular matrix as a scaffold for tissue reconstruction. Cell Develop Biol. 2002;13:377–383.
30. Falanga V, Margolis D, Alvarez O, et al. Rapid healing of venous ulcers and lack of clinical rejection with an allogenic cultured skin equivalent. Arch Dermatol. 1998;134:293–300.
31. Serena TE. A novel fetal bovine matrix scaffold is rapidly incorporated into animal and human wounds resulting in wound bed stimulation. Wounds. 2005;17:A42.













