A Retrospective, Descriptive Study of Sacral Ulcer Flap Coverage in Nonambulatory Patients with Hypoalbuminemia
Abstract: Deep sacral pressure ulcers in nonambulatory hospitalized patients often are managed using surgical flaps. Reports about the effects of protein status on postoperative healing are inconsistent but surgery often is delayed until serum albumin levels normalize. Considering these conflicting data and the potential effects of delayed closure, the protocol at a Philippine national university hospital was changed to allow for early surgical reconstruction of sacral ulcers in hypoalbuminemic nonambulatory patients.
A retrospective chart review was conducted to evaluate clinical outcomes of 16 nonambulatory patients (10 men, 6 women; average age 54 years, range 18 to 74) with moderate to severe hypoalbuminemia who underwent flap surgery for coverage of their Stage III or Stage IV sacral ulcers within a protocol of interdisciplinary care. Outcomes measured included the number of surgeries needed for coverage and wound complications encountered. Patient average albumin level before flap coverage was 21 g/L (range: 8 to 30 g/L), average sacral ulcer size was 10 cm x 10 cm, patients underwent an average of 2.56 procedures to achieve coverage, and average follow-up period was 11.25 months (range: 3 to 33 months, SD ± 10.4) after surgical closure. Of the 16 flaps, 15 (93.75%) were healed on final follow-up. Six patients (37.5%) had wound-related complications with more complications observed in the younger (<54 years old) patient group (r = 0.516; P = 0.039). Results suggest that with a system of interdisciplinary care and collaboration, sacral ulcer flap surgery can be performed in patients with moderate to severe hypoalbuminemia.
Please address correspondence to: Emmanuel P. Estrella, MD, Microsurgery Unit, Department of Orthopedics, University of the Philippines-College of Medicine, Philippine General Hospital, Taft Avenue, Manila, Philippines 1000; email: estee96@yahoo.com.
Pressure ulcer development is a problem in nonambulatory, hospitalized patients, with varying incidence across institutions. In a prospective cohort study1 of 286 bed- or wheelchair-bound patients age 55 years and older, 12.9% developed Stage II or Stage III pressure ulcers in a median of 9 days following admission. A retrospective study2 of 47 younger traumatic spinal cord injury patients admitted to a university hospital showed 42.5% developed pressure ulcers, 45% of which were Stage III or Stage IV. Surgical debridement and reconstruction have been recommended3,4 for severe pressure ulcers (Stage III and Stage IV), but several retrospective studies4-6 found impaired wound healing and ulcer recurrence, prompting recommendations for strict patient selection — ie, surgical candidates should be medically stable, well-nourished, and capable of participating in postoperative rehabilitation protocols.
 Serum albumin, a basic screening test for protein status, is a gross indicator of patient’s nutrition and fluid balance.7 Although not as sensitive as prealbumin in detecting acute nutritional status change,7 its low cost and easy availability made it the biochemical test of choice for detecting malnutrition in the local setting. Reports on the role of albumin in wound healing are conflicting. Low preoperative serum albumin (<30 g/L) has been reported in hospitalized elderly patients8,9 and has been regarded as an important risk factor for postoperative septic complications across surgical specialties that include orthopedics,10,11 cardiothoracic,12,13 and abdominal.14 Studies15,16 involving prospective cohorts patients (n = 170 and n =103, respectively) undergoing hip or knee joint replacement surgery found that preoperative serum albumin had no significant predictive value for delayed wound healing. Other retrospective studies4-6 suggest that normal albumin levels are a critical factor in successful surgical closure of Stage III and Stage IV sacral ulcers, especially in nonambulatory patients.
Serum albumin, a basic screening test for protein status, is a gross indicator of patient’s nutrition and fluid balance.7 Although not as sensitive as prealbumin in detecting acute nutritional status change,7 its low cost and easy availability made it the biochemical test of choice for detecting malnutrition in the local setting. Reports on the role of albumin in wound healing are conflicting. Low preoperative serum albumin (<30 g/L) has been reported in hospitalized elderly patients8,9 and has been regarded as an important risk factor for postoperative septic complications across surgical specialties that include orthopedics,10,11 cardiothoracic,12,13 and abdominal.14 Studies15,16 involving prospective cohorts patients (n = 170 and n =103, respectively) undergoing hip or knee joint replacement surgery found that preoperative serum albumin had no significant predictive value for delayed wound healing. Other retrospective studies4-6 suggest that normal albumin levels are a critical factor in successful surgical closure of Stage III and Stage IV sacral ulcers, especially in nonambulatory patients.
Per a review of the literature, early surgical closure of Stage III and Stage IV sacral ulcers has been found to reduce local infection, facilitate rehabilitation, and require less skilled wound care.4 Sacral ulcers can be successfully managed with a variety of flaps, most notably musculocutaneous17,18 or fasciocutaneous flaps.18,19 Yet healing of sacral ulcers after flap coverage in a nonambulatory population can be problematic. In a retrospective cohort study, Disa et al20 reviewed postoperative outcomes of 40 patients (mean age 42 years) with 66 pressure ulcers who underwent various types of flap coverage. Of the 40 patients studied, 24 (60%) were paraplegic, 16 out (40%) were nonambulatory due to chronic disabilities, and 21 (52%) experienced flap-related complications postoperatively. Of the 35 patients available for postdischarge follow-up, 69% had an ulcer recurrence after an average of 9.3 months. In another retrospective study, Berry et al21 documented a pressure ulcer recurrence rate of 47% in a population of 41 paraplegic patients (average age 45 years) who underwent rotation flap coverage of various stages of pressure ulcers.
In the authors’ institution, the traditional protocol for improving a patient’s overall health status before surgery involved several weeks of monitored feeding and the administration of intravenous antibiotics. However, considering the conflicting data about the effects of low preoperative serum albumin levels on patient outcomes, the protocol was changed to allow for early surgical reconstruction of sacral ulcers in hypoalbuminemic nonambulatory patients. The purpose of this retrospective descriptive study was to ascertain clinical outcomes of sacral ulcer surgical coverage in nonambulatory patients with moderate to severe hypoalbuminemia, including the number of surgeries required to achieve wound closure and the rate of wound healing complications.
Methods
Patients. A retrospective chart review of patients who underwent flap coverage for sacral ulcers from September 2003 to January 2009 at the Department of Orthopedics’ Hand and Microsurgery unit in a tertiary university hospital in the Philippines was conducted after obtaining approval of the local ethics committee. Charts of patients who were nonambulatory, had Stage III or Stage IV sacral ulcers, a preoperative albumin level of <35 g/L before surgery, and a minimum of 3 months’ postsurgical follow-up were included in the review. Ambulatory patients, patients with ambulatory potential after flap coverage or serum albumin levels >35 g/L, and persons with a history of flap coverage of sacral ulcers were excluded from the study.
Variables. The following variables were extracted from the patient charts and entered into a Microsoft 2004 Excel database: patient age, gender, paralysis type and etiology, presence of comorbid conditions, sacral ulcer stage and size, presence and stage of pressure ulcers in other anatomical locations, preoperative serum albumin, and surgical procedures performed apart from sacral ulcer coverage.
Outcomes. Length of follow-up was noted and outcomes assessed included wound-related complications and number of surgeries needed for final flap closure. The following wound-related complications, as defined by Gherini et al,16 were included: persistent drainage for more than 3 days and wound dehiscence or separation of wound edges by more than 1 cm in width and more than 2 cm in length. Sacral ulcer recurrence and necrosis of the flap or flap edges also were considered wound-related complications.
Patient care. All patients were referred to the Microsurgery Unit for co-management of sacral ulcers by medical, department, and orthopedic spine sections of the same hospital. At the time of referral, all patients were receiving nutritional intervention and intravenous antibiotics. Ulcers were staged using the National Pressure Ulcer Advisory Panel Consensus classification.3,4 Patients with Stage III or Stage IV sacral ulcers were deemed surgical candidates once they received cardiopulmonary clearance to undergo debridement and flap coverage by the medical internists.
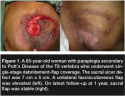 Serum albumin was measured upon referral. Persons with <35 g/L received nutritional intervention consisting of a 25 to 30 kcal/kg body weight and 1 to 1.5 g protein/kg body weight3 diet for an average of 3 weeks with close monitoring. Pressure redistribution by log-rolling on regular hospital mattresses every 3 to 4 hours was strictly enforced; no specialized beds were in place. Daily bedside wound care using moist gauze packs was performed on patients awaiting surgery. Bedside therapy provided by rehabilitation physicians was continued and/or initiated and consisted of passive range-of-motion and strengthening exercises when applicable to encourage self-assisted bed turning. Serum albumin was measured again within 1 week before surgery to determine if albumin levels changed after a high protein diet was implemented. All patients underwent fasciocutaneous rotation V-Y advancement flap coverage for the sacral ulcer as previously described22 (see Figure 1). Surgical coverage included radical debridement of necrotic tissue, padding of bony prominences, delivery of vascularized tissue into the wound with a fasciocutaneous flap, dead space management with a negative suction drain, tension-free closure, and preservation of alternative tissue donors for recurrences. Average operative time was 3 hours with a mean estimated blood loss of 500 cc. Negative suction drains were maintained until output was <50 cc. Intravenous antibiotics were adjusted based on culture and sensitivity results from the infectious disease specialists.
Serum albumin was measured upon referral. Persons with <35 g/L received nutritional intervention consisting of a 25 to 30 kcal/kg body weight and 1 to 1.5 g protein/kg body weight3 diet for an average of 3 weeks with close monitoring. Pressure redistribution by log-rolling on regular hospital mattresses every 3 to 4 hours was strictly enforced; no specialized beds were in place. Daily bedside wound care using moist gauze packs was performed on patients awaiting surgery. Bedside therapy provided by rehabilitation physicians was continued and/or initiated and consisted of passive range-of-motion and strengthening exercises when applicable to encourage self-assisted bed turning. Serum albumin was measured again within 1 week before surgery to determine if albumin levels changed after a high protein diet was implemented. All patients underwent fasciocutaneous rotation V-Y advancement flap coverage for the sacral ulcer as previously described22 (see Figure 1). Surgical coverage included radical debridement of necrotic tissue, padding of bony prominences, delivery of vascularized tissue into the wound with a fasciocutaneous flap, dead space management with a negative suction drain, tension-free closure, and preservation of alternative tissue donors for recurrences. Average operative time was 3 hours with a mean estimated blood loss of 500 cc. Negative suction drains were maintained until output was <50 cc. Intravenous antibiotics were adjusted based on culture and sensitivity results from the infectious disease specialists.
All patients received standard postoperative care. This included pressure redistribution by resting in a prone position when tolerated. Patients were shifted to a lateral position on each side every 3 to 4 hours for 1 to 2 weeks or until wound healing. Persons who could not tolerate resting in the prone or lateral position were placed supine with a large air-inflated “doughnut-shaped” air pillow placed underneath the gluteal area with the sacrum centered in the hole, free from pressure, for 1 to 2 weeks. The pillow was half inflated to prevent hip extension. The surgical site was cleaned daily and after every bowel movement. Bedside therapy was started on the third postoperative day. Sitting was initiated after 3 to 4 weeks. All patients also remained under the care of the Dietary Department.
 Data analysis. Nominal data were summarized using means and percentages. The correlation between patient-related factors (age group, gender, and presence of comorbidity) and outcome (wound-related complication and number of surgeries needed to close the wound) was determined. If the two variables were dichotomous, a phi correlation coefficient was calculated. P <0.05 was considered significant.
Data analysis. Nominal data were summarized using means and percentages. The correlation between patient-related factors (age group, gender, and presence of comorbidity) and outcome (wound-related complication and number of surgeries needed to close the wound) was determined. If the two variables were dichotomous, a phi correlation coefficient was calculated. P <0.05 was considered significant.
Results
Patients. A search of patient census data and a medical record review of all patients who had sacral ulcer flap coverage (n = 25) identified 16 patients (10 men, six women, average age at time of surgery 54 years) who met the inclusion criteria. Of these, 12 were paraplegic, three were quadriplegic, and one was a hemiplegic with a contralateral untreated femoral neck fracture (see Table 1). All patients were nonambulatory and dependent on others for bed mobility. The average follow-up period was 11.25 months (range 3 to 33 months, SD + 10.4 months). Average albumin level before flap coverage was 21 g/L + 5.7 g/L (see Table 1).
Wound etiology. Patients had developed sacral ulcers due to prolonged immobilization as a consequence of spine trauma (n = 5), Pott’s disease (n = 4), cerebrovascular accidents (n = 4), spine tumor (n = 2), and an ossified posterior longitudinal ligament (OPLL) of the cervical spine (n = 1). Five patients had additional comorbidities: one had insulin-dependent diabetes mellitus and four with cerebrovascular disease had hypertensive heart disease.
Wound severity. Fourteen patients had Stage IV and two had Stage II sacral ulcers. The average size of the sacral ulcer before flap coverage was 9 cm x 10 cm. Five patients also had Stage III pressure ulcers in other anatomical locations, specifically greater trochanteric (n = 3) area, ischium (n = 2) and back (thoracolumbar junction, n = 3). Overall, 24 ulcers in these 16 patients were treated surgically.
Surgical procedures. Twelve procedures (including eight additional ulcer coverage, two application of spinal instrumentation, and two releases of contractures) were performed: three greater trochanteric ulcers were managed with rotation flaps and two ischial ulcers were closed primarily. In the three patients with upper back ulcers, two were managed with skin grafting and one was managed with a local rotation flap. Posterior instrumentation for spinal stabilization was performed on two patients 1 month after the sacral ulcer coverage. Two patients underwent release of contractures before sacral ulcer coverage in one stage. Two patients developed corner necrosis of the sacral flap and were managed with local wound suturing.
All but one flap (93.75%) were healed on final follow-up. Sacral ulcer coverage was achieved with an average of 2.56 surgeries per patient. Five patients were provided single-stage debridement/flap coverage; in an additional seven patients, flap closure was achieved during the second surgery. In the remaining four patients, three or more repeated debridements were needed to achieve definitive flap closure due to the persistent infection in the sacral ulcer (see Table 1).
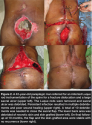
Complications. Six patients (37.5%) had wound-related complications. All wound-related complications involved sacral ulcer flap coverage — two patients had corner necrosis and were managed with primary closure, two had delayed wound healing because of persistent drainage. All six patients eventually healed after delayed flap closure with no further dehiscence or recurrence on latest follow-up (see Figure 2). Two patients had recurrences: one ulcer recurred at 2 months post flap coverage because of poor mobilization and was managed with daily clean, wet-to-dry dressing changes until wound healing. The second recurred at 20 months after flap closure; the patient refused further treatment and eventually died of pulmonary complications.
 No association was found between patients with wound related-complications and the number of surgeries needed for final flap closure. A weak positive association was noted between age group and wound-related complications (r = 0.516; P = 0.039) (see Table 2). Of the eight wound-related complication, more occurred in the younger age group (five, 62.5 %) than the >54 year old age group (one, 12.5%).
No association was found between patients with wound related-complications and the number of surgeries needed for final flap closure. A weak positive association was noted between age group and wound-related complications (r = 0.516; P = 0.039) (see Table 2). Of the eight wound-related complication, more occurred in the younger age group (five, 62.5 %) than the >54 year old age group (one, 12.5%).
Discussion
Currently, no established clinical practice guidelines are available for the management of decubitus ulcers in this Philippine national university hospital. Conservative treatment within the limits of patient’s finances that includes nutritional supplementation, patient turning, and bedside dressing changes is the standard of care for patients with Stage I and Stage II ulcers. When these measures fail to improve Stage III and Stage IV ulcers, surgical treatment of these severe ulcers3,4 is considered. Results of the current study suggest that fasciocutaneous rotation V-Y advancement flap coverage for Stage III and Stage IV sacral ulcers in nonambulatory patients with hypoalbuminemia is a viable option that could result in primary wound healing in 62.5% of patients despite their protein deficiencies. As described in a literature review by Frantz et al4 on the prevention and treatment of decubitus ulcers in nonambulatory patients with hip fractures, early surgical coverage of severe pressure ulcers in nonambulatory patients facilitates early rehabilitation, decreases the susceptibility to infection, and shortens hospital stay.
 In a prospective study of gluteal fasciocutaneous rotation V-Y advancement flap coverage of Stage IV sacral ulcers in 15 patients on prolonged bed rest (seven paraplegic, one quadriplegic, and five with debilitating medical conditions causing prolonged bed confinement; age range 34 to 84 years), Borman and Mural19 reported no one developed wound complications or recurrences in 1.5 to 35 months follow-up. Patient selection criteria (nutritional status an/or rehabilitation potential) was not stipulated. Gluteal fasciocutaneous flaps were found have equivalent rates of healing, healing time, and wound complication rates when compared to the classic myocutaneous flaps in two matched prospective cohorts of nonambulatory elderly patients.18 The ability of a fasciocutaneous flap to cover large defects (maximum of 15 cm x 21 cm)19,22 common in the study author’s setting, along with uncomplicated wound healing,19,22 prompted the adoption of this flap closure technique.
In a prospective study of gluteal fasciocutaneous rotation V-Y advancement flap coverage of Stage IV sacral ulcers in 15 patients on prolonged bed rest (seven paraplegic, one quadriplegic, and five with debilitating medical conditions causing prolonged bed confinement; age range 34 to 84 years), Borman and Mural19 reported no one developed wound complications or recurrences in 1.5 to 35 months follow-up. Patient selection criteria (nutritional status an/or rehabilitation potential) was not stipulated. Gluteal fasciocutaneous flaps were found have equivalent rates of healing, healing time, and wound complication rates when compared to the classic myocutaneous flaps in two matched prospective cohorts of nonambulatory elderly patients.18 The ability of a fasciocutaneous flap to cover large defects (maximum of 15 cm x 21 cm)19,22 common in the study author’s setting, along with uncomplicated wound healing,19,22 prompted the adoption of this flap closure technique.
Gusenoff et al6 reviewed myocutaneous and fasciocutaneous flap coverage in 19 nonambulatory, nonparaplegic elderly patients with Stage III or Stage IV sacral ulcers. Primary healing of a sacral flap was achieved in 47% of patients. This was attributed to selection of motivated patients with clean wounds, controlled spasticity, and a normal albumin and nitrogen balance.
In this case series, primary wound healing of sacral flaps in nonambulatory, predominantly paraplegic, hypoalbuminemic patients was observed in 62.5% of Stage III or Stage IV sacral ulcers with an average of 2.56 surgeries per patient. Although positive protein balance is ideal for uneventful wound healing, a team approach to perioperative care with rehabilitation medicine and nursing departments as recommended by Kierney et al5 could play a crucial role in facilitating primary wound healing after flap coverage. In a retrospective cohort5 of 158 nonambulatory patients (mean age 34.5 years) with 268 Stage III and Stage IV pressure ulcers managed with a collaborative protocol between surgical and rehabilitation teams, overall ulcer recurrence rate was 19% after a mean follow-up of 3.7 years, compared to earlier published rates of 47% to 69%.20,21
Kierney et al’s5 rehabilitation protocol involved use of air-fluidized beds, regular active and passive range-of-motion exercises, patient education on personal hygiene, and sitting and pressure-release maneuvers. This protocol was adapted by the authors’ staff to fit existing hospital conditions. With no available specialized beds, patients tolerated regular turning and the use of cushions to facilitate pressure redistribution. Range-of-motion and strengthening exercises when applicable were taught by physical therapists with the goal of achieving self-assisted pressure-release maneuvers. The surgical team kept the surgical site free from fecal contamination and patient and caregiver education on perineal hygiene was emphasized. The infectious disease expert tailored antibiotic therapy to each patient.
In this case series, the possible causes of complications were diverse. Wound-related complications were associated only with decreasing age (age <54 years old). Disa et al20 noted that 62.5% of their recurrences were patients from the traumatic paraplegic group with a mean age of 32 years. However, this was attributed to patient characteristics rather than age — ie, 50% of these patients sustained penetrating injuries that caused their paraplegia and 75% had a history of drug addiction. Inadequate family support, longer sitting time, and nonadherence to postoperative advice to shift positions every 2 hours were postulated to cause the high recurrence in this group.
All patients under 54 years in the current study were paralyzed because of spine conditions, five (62.5%) of which were traumatic fracture-dislocations. As mentioned by Disa et al,20 lack of family support in a young traumatic spine injury patient could contribute to inadequate pressure redistribution and nursing care at home. This could explain the two recurrences seen in this population. Neither one of the two patients with a recurrence had a comorbid illnesses.
Two cases of flap corner necrosis were attributed to faulty surgical technique (the angles of the corners of the flap were too sharp). The two patients with the largest ulcers had persistent discharge from the surgical sites, possibly attributed to inadequate infection control. The authors believe large defects could have greater bacterial load and bigger resulting dead space when debrided, making infection control more difficult.
Although correction of albumin levels was ongoing from admission, the need to decrease bacterial load with radical debridement and provide early coverage of the deep sacral ulcers was deemed more urgent than albumin level correction. The presence of a large exudative ulcer theoretically would contribute to ongoing protein loss and could increase the risk of septic complications. The authors’ approach to such wounds was that if coverage is possible, correction of protein malnutrition was started but a normal albumin level was not a prerequisite for surgical treatment of pressure ulcers.
Limitations
The authors’ inability to identify a cohort group of nonambulatory patients with Stage III or Stage IV sacral ulcers with normal serum albumin limits the ability to compare outcomes. Because the Microsurgery Unit has been actively co-managing high-grade sacral ulcers for the past 5 years and all patients referred for treatment of their Stage III and Stage IV ulcer were hypoalbuminemic, a control group with optimal albumin levels was not available. The authors maintain a working database on all patients and are still actively collecting data to establish a comparative future study group for their hypoalbuminemic patients.
Conclusion
Within a structure of multidisciplinary care, surgical sacral flap healing can be expected despite suboptimal preoperative albumin level. The authors support the recommendation of aggressive debridement and early flap coverage in medically stable patients with Stage III and Stage IV ulcers. A collaborative approach to patient management among the surgical, medical, rehabilitation, and nursing staff is recommended to facilitate wound healing after surgical coverage.
1. Allman RM, Goode PS, Patrick MM, Burst N, Bartolucci AA. Pressure ulcer risk factors among hospitalized patients with activity limitation. JAMA. 1995;273(11):865–870.
2. Nogueira PC, Caliri MHL, Haas VJ. Profile of patients with spinal cord injuries and occurrence of pressure ulcer at a university hospital. Rev Latino-am Enfermagem. 2006;14(3):372–377.
3. Evans J, Andrews K, Chutka DS, Fleming KC, Garness SL. Pressure ulcers: prevention and management. Mayo Clin Proc. 1995;70:789.
4. Frantz RA. Decubiti prevention and treatment. Tech Orthoped. 2004;19(3):214–222.
5. Kierney PC, Engrav LH, Isik FF, Esselman PC, Cardenas DD, Rand RP. Results of 268 pressure sores in 158 patients managed jointly by plastic surgery and rehabilitation medicine. Plast Reconstr Surg. 1998;102(3):765–772.
6. Gusenoff JA, Redett RJ, Nahabedian MY. Outcomes of surgical coverage of pressure sores in non-ambulatory, nonparaplegic and elderly patients. Ann Plast Surg. 2002;48(6):6633–6640.
7. Hess CT. Monitoring laboratory values: Protein and albumin. In: Hess CT (ed). Clinical Guide: Skin & Wound Care, 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins;2005.
8. Ferguson RP, O’Connor P, Crabtree B, Batchelor A, Mitchell J, Coppola D. Serum albumin and prealbumin as predictors of clinical outcomes of hospitalized elderly nursing home residents. J Am Geriatr Soc. 1993;41:545–549.
9. Reinhardt GF, Myscofski JW, Wilkens DB, Dobrin PB, Mangan JE, Stannard RT. Incidence and mortality of hypoalbuminemic patients in hospitalized veterans. J Parenter Enter Nutr. 1980;11:140–143.
10. Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound healing after amputation. J Bone J Surg. 1984;66(suppl A1):71–75.
11. Kay SP, Moreland JR, Schmitter E. Nutritional status and wound healing in lower extremity amputations. Clin Orthop. 1987;217:253–256.
12. Engelman DT, Adams DH, Byrne JG, et al. Impact of body mass index and albumin on morbidity and mortality after cardiac surgery. J Thorac Cardiovasc Surg. 1999;118:866–873.
13. Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative albumin level as a predictor of operative mortality and morbidity. Arch Surg. 1999;134:36–42.
14. Paocharoen V, Mingphreudhi S, Lertsithichai P, Euanorasetr C. Preoperative albumin levels and postoperative septic complications. Thai J Surg. 2003;24:29–32.
15. Marin LA, Salido JA, Lopez A, Silva A. Preoperative nutritional evaluation as a prognostic tool for wound healing. Acta Orthop Scand. 2002;73(1):2–5.
16. Gherini S, Vaugh BK, Lombardo AV Jr. Delayed wound healing and nutritional deficiencies after total hip arthroplasty. Clin Orthop. 1993;293:188–195.
17. Minami RT, Mills R, Pardo, R. Gluteus maximus myocutaneous flaps for repair of pressure sores. Plast Reconstr Surg. 1977;60:242.
18. Wong TC, Ip FK. Comparison of gluteal fasciocutaneous rotational flaps and myocutaneous flaps for the treatment of sacral sores. Int Orthop. 2006;30:64–67.
19. Borman H, Mural T. The gluteal fasciocutaneous rotation-advancement flap with V-Y closure in the management of sacral pressure sores. Plast Reconstr Surg. 2002;109(7):2325–2329.
20. Disa JJ, Carlton JM, Goldberg NH. Efficacy of operative cure in pressure sore patients. Plast Reconstr Surg. 1992;89(2):272–278.
21. Berry RB. The late results of surgical treatment of pressure sores in paraplegics. Br J Surg. 1980;67:473–474.
22. Ohjimi H, Ogata K, Setsu Y, Haraga I. Modification of the gluteus maximus V-Y advancement flap for sacral ulcers: the gluteal fasciocutaneous flap method. Plast Reconstr Surg. 1996;98(7):1247–1253.













