A Retrospective Data Analysis of Antimicrobial Dressing Usage in 3,084 Patients
Abstract: Knowledge about practice patterns and optimal usage criteria for topical antimicrobial dressings is limited. A retrospective data analysis was conducted to evaluate: 1) the length of time these dressings are applied in a typical episode of wound care, 2) the number of episodes of antimicrobial dressing use, and 3) whether antimicrobial dressings are applied in consideration of signs and symptoms of infection.
Wound care registry data from a level-4 electronic medical record were analyzed, providing information on 3,084 patients older than 17 years seen from July 2003 through December 2008 in 26 hospital-based, outpatient wound centers in 14 states. The 5,541 recorded wounds ranged in size from 0.3 to 225 cm2. One antimicrobial dressing use episode was recorded for 71% of wounds (4.7% had four or more). Mean treatment episode length was 32.5 days (median 21 days). Clinicians used these dressings for a longer period of time if patients had multiple comorbidities (P = 0.0001), a refractory wound (P <0.00001), or were prescribed oral antibiotics (P <0.0002); first dressing use was more common in wounds with signs and symptoms of infection (P <.00001). During an average of 16 (median 10) visits and a follow-up time of 269 days, 61.4% of wounds healed (range 42.2% for flaps or grafts to 67.9% for surgical wounds of all 5,541 wounds). Antimicrobial dressing use for 2 to 4 weeks was associated with a higher proportion of healed wounds, but in wounds that healed, longer dressing use was associated with a longer healing time. The practice pattern observed suggests that antimicrobial dressing usage generally is based on patient and wound assessment variables but prospective studies are needed to develop optimal guidelines of care.
Potential Conflicts of Interest: Dr. Fife has received speaker honoraria from KCI® (San Antonio, TX) and Organogenesis, Inc (Canton, MA) and is a major shareholder in Intellicure, Inc.™ (The Woodlands, TX). Dr. Carter has received speaker honoraria from Hollister Wound Care, LLC (Libertyville, IL). Mr. Walker owns stock in and Mr. Thomson is employed by Intellicure, Inc.
Please address correspondence to: Caroline E. Fife, MD, Department of Medicine, Division of Cardiology, The University of Texas Health Science Center, 6431 Fannin, MSB, Houston, TX 77030; email: Caroline.E.Fife@uth.tmc.edu.
In recent years, the use of antimicrobial dressings that do not contain antibiotics — whether ointments, gels, or impregnated dressings — has increased.1-8 Preclinical studies suggest that when active agents, such as ionic silver, can be released over a period of time at steady concentrations, the likelihood of cytotoxicity is decreased, reducing possible impairment of wound healing while achieving a more constant antimicrobial effect to reduce bacterial growth.1,2 Iodophors (eg, povidone-iodine and cadexomer-iodine, ionic silver, chlorhexidine, and polyhexamethylene-biguanide) are some of the most commonly used antimicrobials,3-8 but the evidence for their efficacy in terms of improving wound healing is limited. Specifically, the sample size of many relevant randomized controlled trials (RCTs) has been small (<140 patients), follow-up is short (≤6 weeks), and evidence about some wound-healing outcomes, such as complete wound healing or time to heal, is frequently lacking although results of recent meta-analyses9,10 of silver-impregnated dressings suggest they improve wound healing and reduce odor, wound pain, and exudate compared to other protocols of care. Regarding wound bacterial control, a body of evidence from different comparative and noncomparative trial designs and in vitro and in vivo studies indicates that early judicious use of antimicrobial dressings can reduce wound bacterial burden or localized infection,3,11-17 although no controlled studies have specifically reported on wound bioburden reduction and improvements in wound healing concurrently.
 Guidelines for antimicrobial usage. Guidelines for prescribing systemic antibiotics often are based on expert opinion rather than scientific fact.18 Also, guidelines for chronic wounds differ substantially because the evidence is far from conclusive, subsequently inviting interpretation. Generating suitable indications for use of topical antimicrobials such as silver-impregnated dressings has been similarly challenging. For example, classic symptoms of wound infection include erythema, heat, pain, edema, and purulence,19 but in infected chronic wounds more reliable indicators proposed by Cutting and Harding20 include serous drainage with concurrent inflammation, delayed healing, discoloration of granulation tissue, pocketing at the base of the wound, friable granulation tissue, malodor, and wound breakdown.
Guidelines for antimicrobial usage. Guidelines for prescribing systemic antibiotics often are based on expert opinion rather than scientific fact.18 Also, guidelines for chronic wounds differ substantially because the evidence is far from conclusive, subsequently inviting interpretation. Generating suitable indications for use of topical antimicrobials such as silver-impregnated dressings has been similarly challenging. For example, classic symptoms of wound infection include erythema, heat, pain, edema, and purulence,19 but in infected chronic wounds more reliable indicators proposed by Cutting and Harding20 include serous drainage with concurrent inflammation, delayed healing, discoloration of granulation tissue, pocketing at the base of the wound, friable granulation tissue, malodor, and wound breakdown.
Using the clinical signs and symptoms checklist, a tool to assess infection in chronic wounds developed by wound care experts and employed by nurses trained in the procedure for the study, Gardner et al21 assessed and quantitatively cultured 36 chronic wounds. Based on sensitivity, specificity, discriminatory power, and positive predictive values, increasing pain, friable granulation tissue, foul odor, and wound breakdown were validated as signs of infection. This confirmed that in chronic wounds, secondary signs of infection were better indicators of infection than classic signs (eg, redness, pain, and heat) because the mean sensitivities were 0.62 and 0.38, respectively. Increasing pain and wound breakdown both were sufficient indicators with a specificity of 100%.
Based on the literature and clinical experience, Sibbald et al22 proposed two paradigms to help clinicians differentiate between critical colonization and infection. The first, NERDS© (nonhealing, exudate, red friable tissue, debris/discoloration, and smell) includes proposed indications for the presence of superficial wound critical colonization; the second, STONEES© (size increasing, temperature elevation, “os” [meaning “probes to bone], new breakdown, erythema/edema, exudate, and smell) summarizes clinical signs of a deep infection. To assess the validity of these paradigms, Woo and Sibbald23 compared semiquantitative culture results (scant or light for NERDS and moderate or heavy growth for STONEES) obtained from 112 patients with 44 leg ulcers and 68 foot ulcers with the appearance (or no appearance) of each sign listed. The authors then calculated the probability of bacterial growth and quantity for each sign, as well as for combinations of signs (two to four) in each paradigm. Sensitivity and specificity, the proportion of ulcers with bacterial growth and one or more clinical signs, and the proportion of ulcers without bacterial growth and without one or more clinical signs were calculated.
Although large differences exist with regard to the sensitivity and specificity of assessment variables between the studies of Gardner et al21 and Woo and Sibbald,23 results for the following clinical variables were similar: debris (NERDS), smell, (NERDS and STONEES), periwound temperature, and new wound breakdown (STONEES). Moreover, sensitivity and specificity were highest when three of the clinical signs to assess bacterial growth were used — 73.3% and 80.5% (NERDS) and 90% and 69.4% (STONEES), respectively. Although resulting information is encouraging, more studies are needed to assess the predictive validity of these assessment variables in the context of bacterial growth on wounds.
 With regard to the optimal length of time an antimicrobial dressing should be applied to achieve the desired result, the most data available address silver-impregnated dressings. The majority of RCTs have such dressings employed for 4 weeks,9,10 but no RCT study to investigate optimal treatment duration has been conducted. Furthermore, results of a literature search going back to 1990 and including PubMed, Scopus, and Cochrane databases, as well as the journals Wounds and World Wide Wounds, using the terms antimicrobial dressing, silver dressing, povidone-iodine, cadexomer-iodine, chlorhexidine, and polyhexamethylene-biguanide suggest that large studies (N = 3,000 or larger) of antimicrobial dressing usage have not been conducted and that information about their use in clinical practice is limited.
With regard to the optimal length of time an antimicrobial dressing should be applied to achieve the desired result, the most data available address silver-impregnated dressings. The majority of RCTs have such dressings employed for 4 weeks,9,10 but no RCT study to investigate optimal treatment duration has been conducted. Furthermore, results of a literature search going back to 1990 and including PubMed, Scopus, and Cochrane databases, as well as the journals Wounds and World Wide Wounds, using the terms antimicrobial dressing, silver dressing, povidone-iodine, cadexomer-iodine, chlorhexidine, and polyhexamethylene-biguanide suggest that large studies (N = 3,000 or larger) of antimicrobial dressing usage have not been conducted and that information about their use in clinical practice is limited.
The purpose of this retrospective study was to determine: 1) length of time antimicrobial dressings are applied in a typical episode of wound care, 2) number of episodes of antimicrobial dressing use that occur in wound healing, and 3) whether antimicrobial dressings are applied in consideration of signs and symptoms of infection in persons <17 years old from information in a national wound database.
Methods
Extraction of datasets. Facilities utilizing Intellicure’s (The Woodlands, TX) proprietary electronic medical record (EMR) software can choose to contribute data to the Intellicure Research Consortium (IRC), a national wound registry. The 50 facilities currently using the software are located in 22 US states; they are either stand-alone or hospital-based wound care clinics and utilize their own protocols of care. The software maintains a database of level-4 EMR wound-care data under the auspices of a Health Insurance and Portability and Accountability Act (HIPAA) agreement, updated nightly using a secure file transfer protocol (FTP). The database contains all patient-related information, including wound characteristics, staging system used, treatments provided, facility charges, physician level of service, tests, medications, interventions, and information on patient comorbidities and lifestyle choices (eg, tobacco or alcohol use). Data from facilities participating in the IRC are de-identified and moved to a separate server. The combined database served as the initial reservoir of data for this study, representing 8,335 patients with 21,595 wounds seen from July 2003 through December 2008 in 26 hospital-based, outpatient wound centers in 14 states.
 Study variables. The IRC wound registry was queried using Microsoft’s SQL programmable relational database management system to provide specific datasets; the term query here refers to use of program commands to delineate specific variables (eg, female patients with surgical wounds age 60 to 70 years). For this study, the following independent variables were obtained for all wounds in which an antimicrobial dressing, based on known dressing categories (see Table 1), was used: patient age and gender; number of antibiotic prescriptions, wound cultures, comorbid conditions for each patient during the study period; type of medical insurance; wound type, area, and volume; age of wound before first being seen at a clinic; drainage characteristics (amount, type of drainage); and periwound characteristics. Outcome (dependent) variables included percentage of wounds that healed and time to healing for wounds that healed using clinic visit dates and outcome data.
Study variables. The IRC wound registry was queried using Microsoft’s SQL programmable relational database management system to provide specific datasets; the term query here refers to use of program commands to delineate specific variables (eg, female patients with surgical wounds age 60 to 70 years). For this study, the following independent variables were obtained for all wounds in which an antimicrobial dressing, based on known dressing categories (see Table 1), was used: patient age and gender; number of antibiotic prescriptions, wound cultures, comorbid conditions for each patient during the study period; type of medical insurance; wound type, area, and volume; age of wound before first being seen at a clinic; drainage characteristics (amount, type of drainage); and periwound characteristics. Outcome (dependent) variables included percentage of wounds that healed and time to healing for wounds that healed using clinic visit dates and outcome data.
The number of days an antimicrobial dressing was applied (days of antimicrobial dressing) was calculated by noting the date of first use and last use in a particular wound. Because the presence of an infection cannot be confirmed without quantitative culture results, noted clinician concerns about infection and surrogate measures were collected that might suggest the presence of infection or heavy contamination. The following surrogates of infection were collected for the development of a surrogate infection factor variable: wound culture taken; antibiotics prescribed the day of first treatment (>99% of wounds); wound drainage (green, malodorous, or purulent); periwound characteristics noted to be erythematous; and patient temperature noted to be higher than a specified temperature. The surrogate infection factor was the sum of these five entries (when included in the electronic health record — EHR) when the wound was evaluated; its score could vary from 0 (no signs) to 5 (maximum).
Refinement of datasets. A complete list of antimicrobial dressing products was identified by name (see Table 1); subsequently, the de-identified registry comprising 8,335 patients with 21,595 wounds was initially used to identify wounds that had received those dressings (approxima tely 49.9%). Records for 319 patients aged ≤17 years were excluded from the patient dataset, leaving 21,276 wounds. Cases associated with burns and other types of ulcers — eg, drained abscesses, sutured wounds, malignant lesions (such as primary or metastatic cancers), and ulcerations due to radiation — as well as unidentified types of wounds (n = 1,189) — were deleted from the wound dataset, leaving 20,087 wounds to be analyzed. Ninety cases with negative numbers for days before antimicrobial dressing (a nonsensical number likely due to incorrect data entry) were noted and records that included these cases were deleted and cases with days of antimicrobial dressings = 0 also were excluded from data analysis.
tely 49.9%). Records for 319 patients aged ≤17 years were excluded from the patient dataset, leaving 21,276 wounds. Cases associated with burns and other types of ulcers — eg, drained abscesses, sutured wounds, malignant lesions (such as primary or metastatic cancers), and ulcerations due to radiation — as well as unidentified types of wounds (n = 1,189) — were deleted from the wound dataset, leaving 20,087 wounds to be analyzed. Ninety cases with negative numbers for days before antimicrobial dressing (a nonsensical number likely due to incorrect data entry) were noted and records that included these cases were deleted and cases with days of antimicrobial dressings = 0 also were excluded from data analysis.
Only wounds with complete initial wound size data were selected in the wound encounter database. A data trim was performed to select only those wounds measuring between 0.3 cm2 and 225 cm2, with the rationale that wounds <0.3 cm2 are too small and that the largest venous ulcers would be 15 cm x 15 cm. Very large wounds, which comprised 3% to 5% of the wound databases, often have been found to have undue influence over outcome parameters; thus, they were not included. The wound and wound encounter datasets were merged so each wound had wound size parameters as well days of antimicrobial dressing parameters. Any records that did not have a wound type description were excluded, leaving 5,643 wound records in the database to be analyzed associated with 3,084 patients, approximately 26% of the original wound sample.
Gap analysis. Most patients followed in outpatient wound centers require relatively frequent visits (eg, bimonthly). Total product use was estimated based on the frequency of planned dressing changes from the physician orders (eg, “change dressings daily”) until the next visit. However, if patients failed to return to clinic for follow-up for an extended period, it was not appropriate to assume that antimicrobial dressing use continued without clinical supervision over some long and unobserved time frame. Thus, to identify patients who may have had long “gaps” in their follow-up care, a gap analysis was performed.
 The process examined the merged (final) wound dataset and determined how many visits were associated with each wound (wound type); determined the number of visits during which antimicrobial dressings were applied and removed in a subsequent visit; calculated the number of antimicrobial dressing episodes, defined as application of antimicrobial dressings for a period of time followed by a period of time with no antimicrobial dressings over the course of the wound for each wound; and calculated the number of days the antimicrobial dressings were applied for each episode. This resulted in calculation of two variables: 1) number of antimicrobial dressing episodes per wound, and 2) mean number of days of antimicrobial dressing per episode.
The process examined the merged (final) wound dataset and determined how many visits were associated with each wound (wound type); determined the number of visits during which antimicrobial dressings were applied and removed in a subsequent visit; calculated the number of antimicrobial dressing episodes, defined as application of antimicrobial dressings for a period of time followed by a period of time with no antimicrobial dressings over the course of the wound for each wound; and calculated the number of days the antimicrobial dressings were applied for each episode. This resulted in calculation of two variables: 1) number of antimicrobial dressing episodes per wound, and 2) mean number of days of antimicrobial dressing per episode.
The dataset then was refined to ensure each wound had matching gap and other variable-associated data. Then, the dataset was split and recombined to ensure proper variable continuity, leaving 3,084 patients with 5,541 wounds.
Statistical analysis. Statistical analysis was conducted using SPSS version 16.0 (SPSS, Inc., Chicago, IL). Because mean days of antimicrobial dressing per episode, wound areas and volumes, and time to heal all had non-normal distributions, the data were log transformed before analysis. ANOVA was conducted using the post-hoc Games Howell test for multiple comparisons because Levine’s, Welch’s, and Brown and Forsythe’s tests all showed significance with regard to unequal variance and sample size. When comparing differences between two groups, a t-test was employed.
Correlations between pairs of variables were calculated to obtain Pearson’s correlation coefficient, assuming linear relationships. Cross-tabulations were analyzed using chi-square for nominal level variables and the gamma statistic for ordinal level variables.
To examine the infection surrogate factor, a null hypothesis was developed: antimicrobial dressings are applied without consideration of infection surrogate variables on the day that a wound was examined. The null hypothesis was tested by comparing a dichotomous outcome (ie, was the patient given an antimicrobial dressing?) against the infection surrogate score with scores of ≥3 recoded to 3 (ie, a 2 x 4 matrix) using the gamma statistic.
A univariate analysis of time to heal was conducted using wound type, number of antimicrobial dressing episodes, and infection surrogate score (first visit) as factors and age, number of comorbidities, log mean duration of antimicrobial dressing, and age of wound before first antimicrobial dressing as covariates. Models included main effects and all two-way interactions between factors and covariates and were refined by including only significant variables. 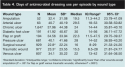
Results
Patient demographics. Of the 3,084 patients analyzed in the study, 50.6% were women. The mean age was 60.1 years (range 18 to 85 years, standard deviation [SD] 16.80) and the mean number of antibiotic prescriptions, wound cultures, and comorbid conditions that each patient had during the study period was 9.1 (range 0 to 77, SD 7.73), 2.3 (range 0 to 80, SD 5.53), and 12.3 (range 1to 43, SD 7.70), respectively. Only 0.5% of the patients paid all expenses out-of-pocket; 43.9% had private insurance and 55.6% had Medicare/Medicaid. The mean number of visits was 16.0 (range: 1 to 294, SD 20.2) and the median number of visits was 10.
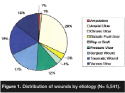
Wound characteristics. The largest category of wounds represented was chronic wounds (n = 1,647), followed by traumatic (n = 974) and surgical wounds (n = 928) (see Figure 1). The mean age of the wound before first examination (visit) was 199.8 days (median 30 days) (see Table 2). Mean wound age before first application of an antimicrobial dressing varied substantially from 60.8 days (SD 95.35), 53.2 days (SD 178.42), and 70.2 days (SD 254.12) for amputation, surgical and traumatic wounds, respectively, to 666 days (SD 2,845.1) for flaps and grafts (see Figure 2).
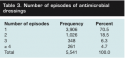
 Antimicrobial dressing use. The majority of wounds (70.5%) had only one episode of antimicrobial dressing usage, with four or more episodes constituting only 4.7% of all wounds (see Table 3). The mean number of antimicrobial dressing usage days per episode (nonlog-transformed data) was 32.5 days (range 1 to 510 days, median 21 days, SD 36.53 days, 95% confidence interval 31.55–33.48 days). When mean days of antimicrobial dressing per episode were examined by wound type, surgical and traumatic wounds were found to have a significantly lower number of days compared to all other types of wounds except amputation wounds (P <0.0001) and traumatic wounds versus flaps and grafts (see Table 4). The number of antimicrobial dressing use episodes was also higher for arterial ulcers and flaps and grafts than for other types of wounds (P <0.001) (see Table 5).
Antimicrobial dressing use. The majority of wounds (70.5%) had only one episode of antimicrobial dressing usage, with four or more episodes constituting only 4.7% of all wounds (see Table 3). The mean number of antimicrobial dressing usage days per episode (nonlog-transformed data) was 32.5 days (range 1 to 510 days, median 21 days, SD 36.53 days, 95% confidence interval 31.55–33.48 days). When mean days of antimicrobial dressing per episode were examined by wound type, surgical and traumatic wounds were found to have a significantly lower number of days compared to all other types of wounds except amputation wounds (P <0.0001) and traumatic wounds versus flaps and grafts (see Table 4). The number of antimicrobial dressing use episodes was also higher for arterial ulcers and flaps and grafts than for other types of wounds (P <0.001) (see Table 5).
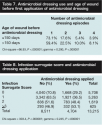
Correlations between days of antimicrobial dressing use per episode, wound size, and volume were poor or nonexistent (r = 0.074, and r = 0.004). A similar result was observed between mean days of antimicrobial dressing per episode and patient age. The correlation between number of patient comorbidities and mean days of antimicrobial dressing use per episode was significant (r = 0.181, P <.00001). When number of patient comorbidities was grouped, the differences between groups were all significant (P <0.0001) (see Table 6). The mean and median number of days of  antimicrobial dressing use per episode was higher in patients with more comorbid conditions. Correlation coefficients between mean days of antimicrobial dressing use per episode, number of antibiotic prescriptions, and number of wound cultures were also significant (r = 0.125 and r = 0.212, respectively; P <0.001). When antibiotic prescriptions were grouped by number (0 to 4, 9 to 12, >12) differences between prescription groups and mean days of antimicrobial dressing per episode were all significant (24.6, 28.8, 36.0, and 44.5 days, respectively; P <0.0002). Finally, when data were grouped by age of wound before first antimicrobial dressing (≤150 days and >150 days), mean days of antimicrobial dressing use per episode for the two groups differed significantly (≤150 days: 30.0 days; >150 days: 43.6 days; P <0.00001) and a higher proportion of wounds that were >150 days old at the time of first antimicrobial dressing received more than one dressing (P <0.00001, chi square and gamma statistic) (see Table 7).
antimicrobial dressing use per episode was higher in patients with more comorbid conditions. Correlation coefficients between mean days of antimicrobial dressing use per episode, number of antibiotic prescriptions, and number of wound cultures were also significant (r = 0.125 and r = 0.212, respectively; P <0.001). When antibiotic prescriptions were grouped by number (0 to 4, 9 to 12, >12) differences between prescription groups and mean days of antimicrobial dressing per episode were all significant (24.6, 28.8, 36.0, and 44.5 days, respectively; P <0.0002). Finally, when data were grouped by age of wound before first antimicrobial dressing (≤150 days and >150 days), mean days of antimicrobial dressing use per episode for the two groups differed significantly (≤150 days: 30.0 days; >150 days: 43.6 days; P <0.00001) and a higher proportion of wounds that were >150 days old at the time of first antimicrobial dressing received more than one dressing (P <0.00001, chi square and gamma statistic) (see Table 7).
For the initial visits, 0.6% of patients had an infection surrogate score of 4 or 5. When the infection surrogate score was cross-tabulated against whether an antimicrobial dressing was received on the date of the assessment, the proportion of patients receiving an antimicrobial dressing was higher for wounds with a higher infection score. (P <0.00001) (see Table 8).
 Healing outcomes. During an average of 269 days of care, approximately 61% of all wounds were healed; arterial ulcers, flaps or grafts, and pressure ulcers had the lowest percentage of healed wounds (44.0%, 42.2%, and 47.0%, respectively) (see Table 9). Of the 61% of all wounds that healed, time to healing ranged from 52.8 days for surgical and 89.6 days for traumatic wounds to 377.3 days for arterial ulcers (see Table 10). A poor-to-fair correlation between time to healing and mean days of antimicrobial dressing use (r = 0.404, P = <.000001) was found, with healing taking longer when antimicrobial dressings were applied for a longer period of time. Regarding number of antimicrobial dressing episodes, a monotonic relationship was found between the percentage of wounds healed and the number of episodes (1: 64.6%, 2: 58.1%, 3: 47.7%, and >3: 44.8%; P <.000001), indicating that the more episodes of antimicrobial dressings received, the poorer the outcome.
Healing outcomes. During an average of 269 days of care, approximately 61% of all wounds were healed; arterial ulcers, flaps or grafts, and pressure ulcers had the lowest percentage of healed wounds (44.0%, 42.2%, and 47.0%, respectively) (see Table 9). Of the 61% of all wounds that healed, time to healing ranged from 52.8 days for surgical and 89.6 days for traumatic wounds to 377.3 days for arterial ulcers (see Table 10). A poor-to-fair correlation between time to healing and mean days of antimicrobial dressing use (r = 0.404, P = <.000001) was found, with healing taking longer when antimicrobial dressings were applied for a longer period of time. Regarding number of antimicrobial dressing episodes, a monotonic relationship was found between the percentage of wounds healed and the number of episodes (1: 64.6%, 2: 58.1%, 3: 47.7%, and >3: 44.8%; P <.000001), indicating that the more episodes of antimicrobial dressings received, the poorer the outcome.
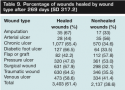 When mean days per antimicrobial dressing episode was categorized into ≤14 days versus >14 days, a smaller percentage of wounds healed when antimicrobial dressings were applied for a shorter duration (59.3% versus 62.5%, P = 0.019). The difference was similar for most wound types except for flaps and grafts and traumatic wounds in which the differences were much larger (32% versus 8% and 59.6% versus 68.4%, respectively). Grouped into four categories of antimicrobial dressing use duration, the proportions healed were 59.3% for ≤2 weeks of usage; 64.8% healed for 2 to 4 weeks, 61.4% healed for 4 to 6 weeks, and 60.7% for ≥6 weeks (ie, not statistically significant). One-way ANOVA of time to healing using the same dressing duration periods showed a monotonic increase in time to heal as the duration of antimicrobial dressing increased: ≤2 weeks: 54.6 days; 2 to 4 weeks: 78.7 days; 4 to 6 weeks: 106.7 days; ≥6 weeks: 217.5 days (P <0.001, except between 2 to 4 weeks and 4 to 6 weeks, where P = 0.006).
When mean days per antimicrobial dressing episode was categorized into ≤14 days versus >14 days, a smaller percentage of wounds healed when antimicrobial dressings were applied for a shorter duration (59.3% versus 62.5%, P = 0.019). The difference was similar for most wound types except for flaps and grafts and traumatic wounds in which the differences were much larger (32% versus 8% and 59.6% versus 68.4%, respectively). Grouped into four categories of antimicrobial dressing use duration, the proportions healed were 59.3% for ≤2 weeks of usage; 64.8% healed for 2 to 4 weeks, 61.4% healed for 4 to 6 weeks, and 60.7% for ≥6 weeks (ie, not statistically significant). One-way ANOVA of time to healing using the same dressing duration periods showed a monotonic increase in time to heal as the duration of antimicrobial dressing increased: ≤2 weeks: 54.6 days; 2 to 4 weeks: 78.7 days; 4 to 6 weeks: 106.7 days; ≥6 weeks: 217.5 days (P <0.001, except between 2 to 4 weeks and 4 to 6 weeks, where P = 0.006).
 Univariate analysis. After two refinements, the final model included two factors, one covariate, and nine interactions (R2 = 0.598; see Table 11). Although some violation of the homogeneity of covariate regression coefficients was likely, the data clearly show that the more episodes of antimicrobial treatment, the longer it took for wounds to heal after adjustment for mean duration of antimicrobial treatment (see Figure 3). Selecting only data for one episode of antimicrobial treatment, a similar model was developed that used one factor, two covariates, and eight interactions (R2 = 0.497; see Table 12). After adjusting for mean duration of antimicrobial treatment and age of wound before first application of antimicrobial dressings, time to heal by wound type showed that arterial ulcers took the longest time to heal (average 120.2 days) but pressure ulcers also took a long time (average 54.7 days) compared to other wound types (see Table 13).
Univariate analysis. After two refinements, the final model included two factors, one covariate, and nine interactions (R2 = 0.598; see Table 11). Although some violation of the homogeneity of covariate regression coefficients was likely, the data clearly show that the more episodes of antimicrobial treatment, the longer it took for wounds to heal after adjustment for mean duration of antimicrobial treatment (see Figure 3). Selecting only data for one episode of antimicrobial treatment, a similar model was developed that used one factor, two covariates, and eight interactions (R2 = 0.497; see Table 12). After adjusting for mean duration of antimicrobial treatment and age of wound before first application of antimicrobial dressings, time to heal by wound type showed that arterial ulcers took the longest time to heal (average 120.2 days) but pressure ulcers also took a long time (average 54.7 days) compared to other wound types (see Table 13). 
Discussion
This data set represents the largest descriptive evaluation of topical antimicrobial dressing use in clinical practice. Currently, guidelines or best practices regarding indications for use and duration of use for topical antimicrobial dressings are lacking.24 Part of the reason is the limited data in terms of efficacy, duration of use, and indications for use on which to base such guidelines. For example, it has been suggested that wound-healing protocols include a 2-week course of antimicrobial dressings where appropriate,24 but most available study data describe outcomes after 4 weeks of application. Thus, it is reasonable to use antimicrobial dressings for a period of time and then evaluate the wound. If positive results are obtained, a further possible application can be contemplated or the dressings  discontinued, while negative results would indicate that no benefit has been obtained.
discontinued, while negative results would indicate that no benefit has been obtained.
In the current study, the average time antimicrobial dressings were applied in any episode of antimicrobial dressing care was 32.5 days (median 21 days), which would suggest that in practice, clinicians prescribe antimicrobial dressings for about 4 weeks, although antimicrobial dressings for acute wounds, such as traumatic and surgical wounds, were prescribed for shorter periods of time. Using infection surrogate variables on the day a wound was examined, the authors’ hypothesis that antimicrobial dressings are applied without consideration of signs/symptoms of infection was rejected. Although the database did not include all the signs and symptoms described in colonization/infection studies,21-23 the infection surrogate factor algorithm was used in several unpublished studies and the authors believe it has some utility in determining whether at a given visit the wound has some signs of infection. It also should be noted that this algorithm has not been formally validated and it is unknown which or how many possible signs of infection should be used. Nevertheless, by determining the score and cross-tabulating this value with whether an antimicrobial dressing was applied, a significant trend toward antimicrobial dressing use was observed when the score was  higher, suggesting that antimicrobial dressings tend to be used with more signs of infection. The observation that 29% of dressing applications occurred when the infection surrogate score was 0 requires further study. This is not necessarily a best practice and suggests that clinicians might be ordering an antimicrobial dressing for prevention rather than treatment purposes. On the other hand, antibiotic usage was high, with an average of 9.1 prescriptions per wound, which suggests that suspected episodes of infection that required systemic intervention were also high in many instances.
higher, suggesting that antimicrobial dressings tend to be used with more signs of infection. The observation that 29% of dressing applications occurred when the infection surrogate score was 0 requires further study. This is not necessarily a best practice and suggests that clinicians might be ordering an antimicrobial dressing for prevention rather than treatment purposes. On the other hand, antibiotic usage was high, with an average of 9.1 prescriptions per wound, which suggests that suspected episodes of infection that required systemic intervention were also high in many instances.
In this study, 61% of all wounds healed after an average of 269 days. Time-to-heal comparisons between studies can be difficult due to different follow-up times and the nature of interventions. To determine if these study results were broadly in line with prior literature results, the authors examined some published studies in which mean times to heal were available. In the current study, 66.7% of diabetic ulcers healed; from the univariate analysis, it took an average of 31.6 days to heal the ulcer, which is compatible with the findings from the literature. For example, Nabuurs-Franssen et al25 found that among 98 patients who had neuropathic diabetic foot ulcers (44% with peripheral arterial disease and 29% of ulcers infected), the application of total contact casting — the gold standard of treatment — resulted in a healing rate of 76% with an average time to heal of 21 days. On the other hand, a trajectory analysis of 160 patients conducted by Robson et al26 showed that among patients that healed, 67% had healed after 33 days, a result very similar to current study findings. With regard to venous ulcers, Blair et al27 compared a four-layer bandage system against traditional adhesive plaster bandaging (part of larger randomized trial of five different dressings), and found that in 84 days, 74.3% of ulcers healed with an average time to heal of 44.1 days, while Bolton et al28 noted an average time to heal of 57 days for venous ulcers in a variety of settings. In the current study, 58.6% of venous ulcers healed in an average of 39.8 days, which is similar, given the different ways in which results have been reported. Thus, current results do not appear to be substantially out of line with regard to findings reported in the literature.
 Although the study was not specifically designed to retrospectively analyze cohorts that received or did not receive antimicrobial dressings with regard to outcomes, the data did suggest a number of trends. First, the proportion of wounds healed was generally lower with antimicrobial dressings applied for 2 weeks or less compared to longer times. This might indicate that 2 weeks is too short a duration for optimal effect. Some evidence also notes that 2 to 4 weeks might be more optimal in terms of outcomes. At the same time, for wounds that healed, time to healing was significantly longer for wounds with longer use of antimicrobial dressings. Variables that significantly affected the use of antimicrobial dressings included type of wound, age of wound before treatment, number of comorbid patient conditions, patient age, the infection surrogate score, duration of antimicrobial treatment, and number of episodes of antimicrobial treatment.
Although the study was not specifically designed to retrospectively analyze cohorts that received or did not receive antimicrobial dressings with regard to outcomes, the data did suggest a number of trends. First, the proportion of wounds healed was generally lower with antimicrobial dressings applied for 2 weeks or less compared to longer times. This might indicate that 2 weeks is too short a duration for optimal effect. Some evidence also notes that 2 to 4 weeks might be more optimal in terms of outcomes. At the same time, for wounds that healed, time to healing was significantly longer for wounds with longer use of antimicrobial dressings. Variables that significantly affected the use of antimicrobial dressings included type of wound, age of wound before treatment, number of comorbid patient conditions, patient age, the infection surrogate score, duration of antimicrobial treatment, and number of episodes of antimicrobial treatment.
After adjusting for patient age, number of comorbid conditions, duration of antimicrobial treatment, and wound age, there still appeared to be differences with regard to the time required for wounds to heal when receiving one episode of antimicrobial treatment. Finally, both cross-tabulated and univariate analyses indicated that a wound took much longer to heal if it received more than one episode of antimicrobial treatment. This finding was consistent for all types of wounds, even after controlling for number of patient comorbidities, wound age, and duration of antimicrobial treatment. Because this study was not designed to establish causality and possible signs and symptoms of infection were captured only at the start of the treatment, it is not known if these wounds exhibited signs of persistent deep tissue infection or if the treatment itself affected healing.
Although cell culture experiments suggest that some antiseptics, such as povidone-iodine, can adversely affect wound-healing, in the case of silver, it is likely a function of concentration.29 Moreover, iodine complexes, such as cadexomer iodine, show no toxicity at appropriate concentrations30 and the most recent randomized controlled trial of a silver-impregnating dressing conducted for 9 weeks was found to be of definite benefit.31 Thus, it seems unlikely that the treatment itself could have caused impaired wound healing.
Alternatively, clinicians might be using antimicrobial dressings as a precaution because they judge the wound to be at high risk of infection. Although prospective studies are needed to confirm current study observations and help develop optimal antimicrobial dressing usage criteria, the study sample size, consistency of observations, and exclusion only of wounds smaller than 225 cm2 increase confidence that the results adequately reflect current clinical practice. The clinicians whose data contributed to the data set have varying levels of knowledge regarding wound infection and are not likely to have the same criteria in mind as they select products. Previous studies have shown that adherence to even well-accepted clinical practice guidelines is highly variable (eg, compression in venous ulcers and offloading of diabetic foot ulcers32).
Conclusion
Data from a database of 5,541 wounds suggest that clinicians are significantly more likely to use antimicrobial dressings for a longer period of time when managing patients with multiple comorbidities or a refractory (“old”) wound and when oral antibiotics are prescribed. The first antimicrobial dressing application is more likely to occur when wounds exhibit signs and symptoms of infection or heavy contamination. Of the 3,084 wounds evaluated, 61.4% healed after an average of 269 days. The average duration of antimicrobial dressing use was 32.5 days, after which some other type of dressing was used. Although antimicrobial dressing use for 2 to 4 weeks was associated with a higher proportion of healed wounds, in wounds that did heal, longer dressing use was associated with a longer healing time.
1. Fong J, Wood F. Nanocrystalline silver dressings in wound management: A review. Int J Nanomedicine. 2006;1(4):441–449.
2. Agarwal A, Weis TL, Schurr MJ, et al. Surfaces modified with nanometer-thick silver-impregnated polymeric films that kill bacteria but support growth of mammalian cells. Biomaterials. 2010;31(4):680–690.
3. White RJ, Cutting K, Kingsley A. Topical antimicrobials in the control of wound bioburden. Ostomy Wound Manage. 2006;52(8):26–58.
4. Khan MN, Naqvi AH. Antiseptics, iodine, povidone iodine and traumatic wound cleansing. J Tissue Viability. 2006;16(4):6–10.
5. Leaper DJ, Durani P. Topical antimicrobial therapy of chronic wounds healing by secondary intention using iodine products. Int Wound J. 2008;5(2):361-368.
6. Leaper DJ. Silver dressings: their role in wound management. Int Wound J. 2006;3(4):282–294.
7. Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: expanding the armamentarium for infection control and prevention. Clin Infect Dis. 2008;46(2):274–281.
8. Landis SJ. Chronic wound infection and antimicrobial use. Adv Skin Wound Care. 2008;21(11):531–542.
9. Carter MJ, Tingley-Kelley K, Warriner RA III. Silver treatments and silver-impregnated dressings for the healing of leg wounds and ulcers: a systematic review and meta-analysis. J Am Acad Dermatol. 2010: In press.
10. Lo SF, Chang CJ, Hu WY, Hayter M, Chang YT. The effectiveness of silver-releasing dressings in the management of non-healing chronic wounds: a meta-analysis. J Clin Nurs. 2009;18(5):716–728.
11. Sundberg J, Meller R. A retrospective review of the use of cadexomer iodine in the treatment of chronic wounds. Wounds. 1997;9(3):68–86.
12. Hermans MH, Bolton L. How do we manage critically colonized wounds? Rehabil Nurs. 2004;29(6):187–194.
13. Verdú Soriano J, Rueda López J, Martínez Cuervo F, Soldevilla Agreda J. Effects of an activated charcoal silver dressing on chronic wounds with no clinical signs of infection. J Wound Care. 2004;13(10):419–423.
14. Akiyama H, Oono T, Saito M, Iwatsuki K. Assessment of cadexomer iodine against Staphylococcus aureus biofilm in vivo and in vitro using confocal laser scanning microscopy. J Dermatol. 2004;31(7):529–534.
15. Mertz PM, Oliveira-Gandia MF, Davis SC. The evaluation of a cadexomer iodine wound dressing on methicillin-resistant Staphylococcus aureus (MRSA) in acute wounds. Dermatol Surg. 1999;25(2):89–93.
16. Danielsen L, Cherry GW, Harding K, Rollman O. Cadexomer iodine in ulcers colonised by Pseudomonas aeruginosa. J Wound Care. 1997;6(4):169–172.
17. Hauser J, Rossbach O, Vogt PM, et al. [Efficacy of treatment with Repithel and Jelonet in comparison to treatment with Jelonet alone - a randomized clinical trial in patients receiving meshed skin grafts]. Zentralbl Chir. 2006;131(4):315–321.
18. Howell-Jones RS, Wilson MJ, Hill KE, Howard AJ, Price PE, Thomas DW. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J Antimicrob Chemother. 2005;55(2):143–149.
19. Stotts NA, Hunt TK. Managing bacterial colonization and infection. Clin Geriatr Med. 1997;13(3):565–573.
20. Cutting KF, Harding KG. Criteria for identifying wound infection. J Wound Care. 1994;3(4):198–201.
21. Gardner SE, Frantz RA, Doebbeling BN. The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair Regen. 2001;9(3):178–186.
22. Sibbald RG, Woo K, Ayello EA. Increased bacterial burden and infection: the story of NERDs and STONES. Adv Skin Wound Care. 2006;19(8):447–463.
23. Woo KY, Sibbald RD. A cross-sectional validation study of using NERDS and STONEES to assess bacterial burden. Ostomy Wound Manage. 2009;55(8):40–48.
24. Templeton S. Management of chronic wounds: the role of silver-containing dressings. Primary Intention. 2005;13(4):170–179.
25. Nabuurs-Franssen MH, Sleegers R, Huijberts MS, et al. Total contact casting of the diabetic foot in daily practice: a prospective follow-up study. Diabetes Care. 2005;28(2):243-247.
26. Robson MC, Hill DP, Woodske ME, Steed DL. Wound healing trajectories as predictors of effectiveness of therapeutic agents. Arch Surg. 2000;135(7):773-777.
27. Blair SD, Wright DD, Backhouse CM, Riddle E, McCollum CN. Sustained compression and healing of chronic venous ulcers. BMJ. 1988;297(6657):1159-1161.
28. Bolton L, McNees P, van Rijswijk L, et al. Wound-healing outcomes using standardized assessment and care in clinical practice. J WOCN. 2004;31(2):65-71.
29. Thomas GW, Rael LT, Bar-Or R, et al. Mechanisms of delayed wound healing by commonly used antiseptics. J Trauma. 2009;66(1):82-91.
30. Zhou LH, Nahm WK, Badiavas E, Yufit T, Falanga V. Slow release iodine preparation and wound healing: in vitro effects consistent with lack of in vivo toxicity in human chronic wounds. Br J Dermatol. 2002;146(3):365-374.
31. Dimakakos E, Katsenis K, Kalemikerakis J, et al. Infected venous leg ulcers: management with silver-releasing foam dressing. Wounds. 2009;21:4-8.
32. Fife CE, Carter MJ, Walker D. Why is it so hard to do the right thing in wound care? Wound Repair Regen. 2010; Feb 16 [epub ahead of print].













