A Randomized, Double-Blind, Placebo-Controlled Trial to Determine the Effects of Topical Insulin on Wound Healing
Abstract
Although the literature contains evidence demonstrating the beneficial effects of insulin on wound healing, no suitable method for the routine administration of insulin has been reported. A randomized, double-blind, placebo-controlled trial was conducted to determine the safety and efficacy of topical insulin on healing in 45 patients (29 men, mean age for both groups 40.62 years, range 12 to 71 years) with noninfected acute and chronic extremity wounds.
Patients were randomly assigned to twice-daily topical application (spray) of 1 cc saline 0.9% for each 10 cm2 of wound with or without 10 units (0.1 cc) of insulin crystal and insulin. The endpoint was complete wound closure. Systemic glucose levels were measured before and 1 hour after treatment application. No patients developed signs or symptoms of hypoglycemia and glucose levels pre- and post-application did not differ significantly. Time to healing did not differ significantly between treatment groups. Healing rates were affected by baseline wound area, patient age, wound type (acute versus chronic), and treatment group. The mean rate of healing rate was 46.09 mm2/day in the treatment and 32.24 mm2/day in the control group (P = 0.029), independent of baseline wound size. In this study, the topical application of insulin was safe and effective. Clinical studies with a larger sample size and that include patients with diabetes mellitus are warranted.
Potential Conflicts of Interest: Drs. Rezvani and Shabback received a research grant from EXIR Pharmacological, Iran.
Please address correspondence to: Omid Rezvani MD, Department of Plastic Surgery and Burn Care, Esfahan University of Medical Sciences, 79 Sarabchi, Molasadra St, Esfahan, Iran; email: omidrezvani@aol.com.
Since Bunting’s discovery of insulin in 1921,1 many benefits beyond blood glucose regulation have been documented.2-4 Preclinical and clinical studies have demonstrated positive effects of insulin on wound healing,5-10 but no suitable method for routine clinical use of topically applied insulin has been reported.
Wound healing is a complex biological process influenced by several agents such as insulin-like growth factor (IGF) and human acidic fibroblast growth factor (rh-aFGF).11 In vivo studies have shown that IGF can stimulate the proliferation and differentiation of endothelial cells and fibroblasts and promote granulation tissue regeneration to contribute to wound healing.12-14
Many therapeutic methods are available to effect wound healing such as skin grafts, hydrocolloid dressings, and high-protein diets, but some may not be economically suitable for the patient and/or may be associated with complications.15-18 Similarly, the cost of methods such as negative pressure wound therapy may be prohibitive for many patients.20-28 A less clinically and economically complicated approach to healing chronic wounds seems necessary.
 The purpose of this controlled clinical study was to evaluate the safety and efficacy of topical insulin in patients with acute and chronic wounds.
The purpose of this controlled clinical study was to evaluate the safety and efficacy of topical insulin in patients with acute and chronic wounds.
Materials and Methods
Setting and population. This study was conducted at Esfahan University of Medical Sciences and affiliated hospitals (Department of Dermatology, Alzahra, Musa-Kazem, and Shariati hospitals) and private clinics over a period of 18 months (April 2006 to October 2007). Forty-five consecutive in- and outpatients with skin wounds attending these hospitals and private clinics were enrolled in the study. Patients with acute (crush wounds, burns) and chronic wounds (pressure ulcers) of the upper and lower extremities were eligible to participate. Patients with uncontrolled wound bleeding, severe infection (as determined by the presence of visible pus, wound exudate, redness, or warmth of the wound border; no wounds had visible pus or exudate), immunodeficiency, age >75 years, or chronic medical conditions (such as diabetes mellitus) were excluded, as were patients who indicated that they could not remain in the study.
The study protocol was approved by the Ethics Committee of Esfahan University of Medical Sciences. After explaining the study to participants, informed consent was obtained from all of the patients.
Study design and procedures. This study was designed as a randomized, double-blind, placebo-controlled clinical trial. After eligibility confirmation, 45 patients were randomly assigned to two groups (A = 23 and B = 22) and demographic variables (age, gender) recorded. Wounds were classified as acute or chronic. Before starting the therapeutic procedure, all wounds were fully washed with physiologic serum (ie, saline 0.9%). Dirty or crusted wounds21 were debrided using a surgical blade (bistori) and local anesthesia. Post-debridement bleeding was controlled using sterile gauze packing. Group A patients received 10 units (0.1 mL) of insulin crystal (manufactured by Iran EXIR Pharmacological Industry) in solution with 1 cc saline 0.9% for each 10 cm2 of wound. The solution was sprayed on the wound surface with an insulin syringe needle, twice daily. Group B patients received the same topical application without insulin. The topical treatment was applied twice daily in both groups, left to dry for 30 minutes, and covered with sterile cotton gauze. All patients were positioned to prevent solution run-off from the wound.
To ensure blinding, the package, appearance of the final working solutions, and application needles were identical and the packages had no labels except a product code. One dermatologist, blinded to the treatment and control groups, performed all procedures on all patients. Neither the patient nor the physician was informed about treatment groups and solutions.
Measurements. An expert physician measured the wound using a sterile transparent paper placed on the wound to mark wound borders. The two largest perpendicular diameters were measured using a ruler (in millimeters). To obtain wound area, these two diameters were multiplied to obtain mm2. A sterile caliper was used to ascertain wound depth. Photographs were taken to record primary wound surface and shape. The condition of the wounds including the presence or absence (yes/no) of granulation tissue, bleeding, pain, infection, and other wound complications or healing factors was assessed and recorded pre-treatment and every 3 days after starting treatment. All data were recorded in the patient’s file.
The end point of the study was complete wound closure. Rate of wound healing was calculated as the difference between the primary and final wound area in mm2 as a function of healing time (in days) and reported as mm2/day.20 A wound was considered fully healed when totally closed and epithelialized.
To assess the side effects of topical insulin (ie, hypoglycemia), blood glucose levels were measured with a glucometer 10 minutes before and 1 hour after application of the topical agent in both groups. Adverse events, including headache and vertigo (due to hypoglycemia) were assessed and recorded.
Statistical analysis. Results from all patients who enrolled in the study (intent-to-treat analysis) were tabulated and expressed as mean and standard deviation. Statistical evaluation of the data was performed using the SPSS version 15 (SPSS, Chicago, IL). Comparisons between groups were performed using the Mann-Whitney U test and comparisons within each group were calculated using the Wilcoxon signed rank test. The linear regression model with enter method was used to determine of correlations between healing rate and variables such as age and wound depth. The correlation between healing rate and age, primary wound area, and depth was analyzed by Spearman coefficient test. All results were considered to be significant at the 5% critical level (P <0.05).
Results
Baseline characteristic of patients. A total of 45 patients were enrolled in this study. All patients had crush injuries or metabolic wounds (pressure  ulcers). Patient characteristics (age, gender) and initial wound depth were similar in the two treatment groups (see Table 1 and Table 2). The mean age of all patients was 40.62 years (range 12 to 71 years) — 41.95 years in group A and 40.22 years in group B. Of the 45 patients, 29 (64.4%) were men (15 men in group A and 14 in group B). Wounds were located on the upper and lower extremities: 37 patients in both groups (82.2%) had lower limb wounds (see Table 1 and Table 2). No wounds were found to be infected.
ulcers). Patient characteristics (age, gender) and initial wound depth were similar in the two treatment groups (see Table 1 and Table 2). The mean age of all patients was 40.62 years (range 12 to 71 years) — 41.95 years in group A and 40.22 years in group B. Of the 45 patients, 29 (64.4%) were men (15 men in group A and 14 in group B). Wounds were located on the upper and lower extremities: 37 patients in both groups (82.2%) had lower limb wounds (see Table 1 and Table 2). No wounds were found to be infected.
Assessment of wound healing. All 45 patients completed the study (achieved wound closure). Mean healing rate was 46.09 mm2/day and 32.24 mm2/day in group A and B, respectively (P = 0.29) (see Table 3).
Wound healing rates were not significantly different between men and women (P = 0.86), wound locations (P = 0.62), or wounds of different depth (P = 0.565). A reverse correlation was found between healing rate and patient age (Spearman’s rho = -0.536, P <0.001) and a positive correlation was observed between wound size and healing rate (Spearman’s rho = 0.745, P <0.01). Crush injury wounds healed faster (48.46 mm2/day) than metabolic disease wounds (24.27 mm2/day, P <0.01).
Because baseline wound size was larger in group A patients, the linear regression test was used to assess the correlation between healing rate and wound surface; healing rate was considered as a dependent variable and “group” and “wound surface” as independent variables. Insulin plus saline had more effect than saline without insulin on healing rate regardless of size (P = 0.029).
Safety evaluation. No significant adverse drug events or reactions were observed and none of the participants experienced adverse systemic effects such as hypoglycemia, hypokalemia, hypoaminoacidemia, vertigo, and headache related to  insulin administration. Blood glucose levels did not differ between treatment groups or following application of the treatment (see Table 4). No wound infection or uncontrolled wound bleeding occurred during the treatment period and none of the subjects had local pain (see Figure 1).
insulin administration. Blood glucose levels did not differ between treatment groups or following application of the treatment (see Table 4). No wound infection or uncontrolled wound bleeding occurred during the treatment period and none of the subjects had local pain (see Figure 1).
Discussion
Wound healing is a complex biologic process that involves chemotaxis and division of cells (neovascularization) that comprises synthesis of extracellular matrix proteins and remodeling of tissues. Sustained skin wounds are more susceptible to bacterial contamination and more likely to foster unfavorable complications; thus, many studies have reported on efforts and products to improve healing rates which may, in turn, reduce complication rates.11-13,21
A review22 of the physiological properties of insulin suggests it might favorably influence wound healing because it can stimulate growth of individual cells as well as cause increased anabolism of the organism as a whole. The amino acid chain in the IGF molecular structure is similar to proinsulin, which is manufactured in the pancreatic Langerhans cells, with 86 amino acids (insulin is 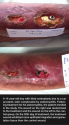 produced when a 35-amino acid chain — C-peptide — detaches from proinsulin25). IGF-1 binds to at least two cell surface receptors: the IGF-1 receptor (IGFR) and the insulin receptor. The IGF-1 receptor seems to be the “physiologic” receptor, binding to IGF-1 at significantly higher affinity than it binds the insulin receptor. Like the insulin receptor, the IGF-1 is a tyrosine kinase receptor — ie, it causes the addition of a phosphate molecule on particular tyrosines.24,25 IGF-1 activates the insulin receptor at approximately 0.1 times the potency of insulin. Part of this signaling may be via the IGF-1R-insulin receptor heterodimers. Binding studies24 show that IGF-1 binds the insulin receptor 100-fold less well than insulin, which does not correlate with the actual potency of IGF-1 in vivo at inducing phosphorylation of the insulin receptor and hypoglycemia.
produced when a 35-amino acid chain — C-peptide — detaches from proinsulin25). IGF-1 binds to at least two cell surface receptors: the IGF-1 receptor (IGFR) and the insulin receptor. The IGF-1 receptor seems to be the “physiologic” receptor, binding to IGF-1 at significantly higher affinity than it binds the insulin receptor. Like the insulin receptor, the IGF-1 is a tyrosine kinase receptor — ie, it causes the addition of a phosphate molecule on particular tyrosines.24,25 IGF-1 activates the insulin receptor at approximately 0.1 times the potency of insulin. Part of this signaling may be via the IGF-1R-insulin receptor heterodimers. Binding studies24 show that IGF-1 binds the insulin receptor 100-fold less well than insulin, which does not correlate with the actual potency of IGF-1 in vivo at inducing phosphorylation of the insulin receptor and hypoglycemia.
In this study, the use of topical insulin was safe and effective. Although wound healing rates were significantly different between the two groups, average time to healing was not, which may have been the result of the observed difference in initial wound area. While not significantly different, wounds in the treatment group were larger at baseline than those in the control group.
In this and other studies, initial wound area correlated with wound healing rate — ie, larger wounds healed at a faster pace than smaller wounds. However, in the current study, the healing rate in the treatment group was higher than in the control group, regardless of initial wound size.
Unlike reported systemic effects of insulin injected into the wound, topical application did not cause a decrease in glucose levels nor were signs and symptoms of hypoglycemia observed.
Limitations and Implications for Future Study
Study limitations include the small patient population (sample size) and the potential limitations of using simple wound measurement methods. Although several methods can be used to more accurately calculate wound size (eg, scanner and computer software), these were not available and the methods used may overestimate or underestimate the actual size of the wounds. However, the consistency of the results suggests that additional studies, using more accurate wound assessment methods and including patients with diabetes mellitus, are warranted. Future studies also should compare the effect of crystal and neutral protamine hagedorn (NPH) insulin on wound healing.
Conclusion
The use of topical insulin was found to be safe and effective in patients with a variety of acute and chronic wounds. Results confirm that topically applied insulin can accelerate wound healing. Its molecular structure is similar to IGF, especially IGF-1, which acts as a growth factor.
Acknowledgments
The authors thank Dr. Esmaeel Shabbak, Dr. Farshid Alaeddini, and Dr. Mohammad Farkhani for funding this research. The authors also thank the EXIR Pharmacological industry for providing the treatment and control treatments.
1. Banting FG, Best CH. The internal secretion of the pancreas. J Lab Clin Med. 1921;7:251–266.
2. Duckworth WC, Fawcett J, Reddy S, Page JC. Insulin-degrading activity in wound fluid. J Clin Endocrinol Metabol. 2004;89:847–851.
3. Mastrogiannis DS, Spiliopoulos M, Mulla W, Homko CJ. Insulin resistance: the possible link between gestational diabetes mellitus and hypertensive disorders of pregnancy. Curr Diab Rep. 2009;9(4):296–302.
4. Fkatsiki N, Georgiadou E, Hatzitolios AI. The role of insulin-sensitizing agents in the treatment of ploycystic ovary syndrome. Drugs. 2009;69(11):1417–1431.
5. Edmunds T. Evaluation of effects of topical insulin on wound healing in distal limb of horse. Vet Med Small Animal Clinician. 1976;71(4):451–457.
6. Hanam SR, Singleton CE, Rudek W. The effect of topical insulin on infected cutaneous ulcerations in diabetic and nondiabetic mice. J Foot Surg. 1983;22(4):298–301.
7. Liu Y, Zhang X, Zhang Z, Fang P-Y, Xu W-S. Effects of topical application of insulin on wound healing in scalded rats. Zhonghua Shao Shang Za Zhi. 2004;20(2):98–101.
8. Liu Y, Zhang X, Zhang Z, Xu W-S. The influence of topical application of insulin on the formation of basement membrane in scalded rats. Zhonghua Shao Shang Za Zhi. 2005;21(6):445–447.
9. Pierre EJ, Barrow RE, Hawkins HK, et al. Effects of insulin on wound healing. J Trauma Inj Infect Crit Care. 1998;44:342.
10. Greenway SE, Filler LE, Greenway LF. Topical insulin in wound healing: a randomized, double-blind, placebo-controlled trial. J Wound Care. 1999;8:526.
11. Ma B, Cheng D-S, Xia ZF. Randomized, multicenter, double blind, and placebo controlled trial using topical recombinant human acidic fibroblast growth factor for deep partial-thickness burns and skin graft donor site. Wound Repair Regen. 2007;15(6):795–799.
12. Sureshbabu A, Okajima H, Yamanaka D, et al. IGFBP-5 induces epithelial and fibroblast responses consistent with the fibrotic response. Biochem Soc Trans. 2009;37(Pt 4):882–885.
13. Feng Y, Visovatti S, Johnson CS, et al. Age and growth factors in porcine full-thickness wound healing. Wound Repair Regen. 2000;9(5):371–377.
14. Wang JM, Hayashi T, Zhang WR, Sakai K, Shiro Y, Abe K. Reduction of ischemic brain injury by topical application of insulin-like growth factor-I after transient middle cerebral artery occlusion in rats. Brain Res. 2000;859(2):381–385.
15. Abramo F, Argiolas S, Pisani G, Vannozzi I, Miragliotta V. Effect of a hydrocolloid dressing on first intention healing surgical wounds in the dog: a pilot study. Austral Vet J. 2008;86(3):96–99.
16. Binda GA, Trizi F. Treatment of unhealed wound after anal fistulotomy with full-thickness skin graft. Tech Coloproctol. 2007;11(3):294.
17. Brown-Etris M, Milne C, Orsted H, et al. A prospective, randomized, multisite clinical evaluation of a transparent absorbent acrylic dressing and a hydrocolloid dressing in the management of Stage II and shallow Stage III pressure ulcers. Adv Skin Wound Care. 2008;21(4):169–174.
18. Pandaitan S, Ilijevski N, Matic P, Gajin P, Radak D. Treatment of the infected wound with exposed silver-ring vascular graft and delayed Thiersch method of skin transplant covering, Srp Arh Celok Lek. 2005;133(1-2):69–71.
19. Steenvoorde P, Oskam J. Commentary on Blake FA, et al. The biosurgical wound debridement: experimental investigation of efficiency and practicability. Wound Repair Regen. 2008;16(3):466.
20. Jessup RL. What is the best method for assessing the rate of wound healing? A comparison of 3 mathematical formulas. Adv Skin Wound Care. 2006;19:138:140–146.
21. Fu X, Li X, Cheng B, Chen W, Sheng Z. Engineered growth factors and cutaneous wound healing: success and possible questions in the past 10 years. Wound Repair Regen. 2005;13:122–130.
22. Apikoglu-Rabus S, Izzettin FV, Turan P. Effect of topical insulin on cutaneous wound healing in rats with or without acute diabetes. Clin Exp Dermatol. 2009; epub ahead of print.
23. Hardman JG, Limbird LE, Gilman AG (eds). The Pharmacological Basis of Therapeutics, 10th edition. New York, NY; McGraw-Hill;2001:1679–1681.
24. Siddle K, Uro B, Niesler CA, et al. Specificity in ligand binding and intracellular signaling by insulin and insulin-like growth factor receptors. Biochem Soc Trans. 2001;29:513–525.
25. De Meyts P, Sajid W, Palsgaard J, et al. Insulin and IGF-1 receptor structure and binding mechanism. In: Saltiel AR, Pessin JE (eds). Mechanism of Insulin action. Austin, TX: Landes Bioscience;2007.
26. Gabriel A, Shores J, Heinrich C, et al. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J. 2008;5(3):399–413.
27. Noble-Bell G, Forbes A. A systematic review of the effectiveness of negative pressure wound therapy in the management of diabetes foot ulcers. Int Wound J. 2008;5(2):233–242.
28. Peinemann F, McGauran N, Sauerland S, Lange S. Disagreement in primary study selection between systematic reviews on negative pressure wound therapy. BMC Med Res Methodol. 2008;8:41.













