A Prospective, Descriptive Pressure Ulcer Risk Factor and Prevalence Study at a University Hospital in Turkey
Introduction
Despite advances in medicine, surgery, and nursing care, pressure ulcers continue to be a common healthcare problem among hospitalized patients,1,2 causing pain, suffering, and frustration. Pressure ulcers increase the workload of healthcare clinicians and as a consequence increase healthcare costs dramatically.3,4 Identifying individuals at risk may help reduce pressure ulcer prevalence.
 Numerous pressure ulcer risk factor and prevalence studies have been conducted in European countries and the US. In Turkey, however, such studies are limited and no national or local database of patient records regarding pressure ulcer prevalence or incidence rates exists nor are guidelines available for developing an assessment tool. In addition, in many Turkish hospitals, patient records do not contain valid and reliable data about pressure ulcers and no specific procedures are in place to promote pressure ulcer prevention. Many hospitals lack written standards of care related to nutrition, hydration, pressure relief, and local wound care. In order to deliver quality care to patients, it is essential that Turkish healthcare professionals gain knowledge of the locations on the body most at risk for pressure ulcer development and general risk factors and preventive measures pertaining to pressure ulcers. Pressure ulcer prevalence rates and the proportion of individuals at risk for pressure ulcer development are needed.
Numerous pressure ulcer risk factor and prevalence studies have been conducted in European countries and the US. In Turkey, however, such studies are limited and no national or local database of patient records regarding pressure ulcer prevalence or incidence rates exists nor are guidelines available for developing an assessment tool. In addition, in many Turkish hospitals, patient records do not contain valid and reliable data about pressure ulcers and no specific procedures are in place to promote pressure ulcer prevention. Many hospitals lack written standards of care related to nutrition, hydration, pressure relief, and local wound care. In order to deliver quality care to patients, it is essential that Turkish healthcare professionals gain knowledge of the locations on the body most at risk for pressure ulcer development and general risk factors and preventive measures pertaining to pressure ulcers. Pressure ulcer prevalence rates and the proportion of individuals at risk for pressure ulcer development are needed.
Literature Review
Pressure ulcers, also known as bed sores and decubitus ulcers, occur mainly in parts of the body subject to high pressure from body weight on bony prominences.5 These ulcers are graded or staged according to the level of tissue damage. The National Pressure Ulcer Advisory Panel (NPUAP)6 grading system includes four stages. A Stage I pressure ulcer is an observable, pressure-related alteration of intact skin whose indicators as compared to an adjacent or opposite area on the body may include changes in one or more of the following: skin temperature (warmth or coolness), tissue consistency (firm or boggy feel), and/or sensation (pain, itching). A Stage II ulcer is defined as an area of partial-thickness skin loss involving the epidermis, dermis, or both that presents clinically as an abrasion, blister, or shallow crater. A Stage III ulceration is an area of full-thickness skin loss involving damage to or necrosis of subcutaneous tissue that may extend down to, but not through, underlying fascia and presents clinically as a deep crater with or without undermining adjacent tissue. A Stage IV ulcer involves full-thickness skin loss with extensive destruction, tissue necrosis, or damage to muscle, bone, or supporting structures (eg, tendon, joint, capsule).6,7
In the clinical setting, prevalence refers to the number of cases measured at one point in time. Lahmann et al2 stated that pressure ulcer prevalence in healthcare facilities in different countries ranged from <5% to >40%. In Germany, these researchers found that the prevalence of pressure ulcers was 11.7% among 10,237 patients in hospitals and 1,347 residents in nursing homes – in all, 11,584 patients and residents in 66 institutions throughout Germany. This study, utilizing a Braden scale (BS) cut-off score of 18 points,2 also found that the proportion of at-risk individuals in hospitals and nursing homes was 29.0%. The Fourth National Pressure Ulcer Prevalence Survey in the US, conducted in 1995 and comprising 265 acute hospitals (patient n = 39,874) found overall prevalence rates of 10.1%.8 Ayello and Braden9 state that according to the NPUAP, overall pressure ulcer prevalence rates in acute care settings range from 10.1% to 17%; whereas, Thoroddsen,10 in a study of 22 Icelandic hospitals (patient n = 642), reported the prevalence of pressure ulcers to be 8.9%. In Sweden, results of a recently published study suggest a pressure ulcer prevalence of 18.5% in acute care and 23.3% in intensive care.11 In addition, the percentage of patients at risk for pressure ulcer development (BS <17) was 19.4% in acute care and 66.7% in intensive care settings.11
In Turkey, reliable statistical information regarding the prevalence of pressure ulcers in hospitals is limited due to a lack of large representative studies. A recent descriptive, cross-sectional study12 reported a prevalence rate of 7.2% in a university hospital setting (n = 922); this rate increased to 9.1% if ophthalmology, psychiatry, gynecology and obstetrics, and pediatric units were excluded from statistical analysis.
Several populations are at increased risk for pressure ulcer development. Generally, immobile patients, older adults, patients with a low serum albumin and body mass index (BMI) or certain disease processes or symptomology (eg, diabetes mellitus, hypertension, respiratory disease, vascular disease, fracture, and incontinence), patients with circulatory impairment, and patients undergoing surgery or in critical care facilities have higher risk for developing pressure ulcers.5,7,13-17 Other risk factors include neurological impairment, diminished mentation, and prolonged surgical procedures under general anesthesia.18 Diabetes is predictive of patient risk for pressure ulcer development related to potential dehydration and/or circulatory problems. In a descriptive study conducted by Lepistö et al13 involving 164 patients with pressure ulcers, it was found that 56% suffered from poor general health, 63% were immobile, and 29% experienced a sense of poor psychological well-being. Lindgren et al19 found that surgical patients with pressure ulcers weighed less and tended to have lower BMIs and lower serum albumin than patients without pressure ulcers.
Purpose
The purpose of this study was to determine the prevalence of pressure ulcers and to identify patient characteristics associated with an increased risk for pressure ulcer development among adult patients in medical and surgical units in a university hospital in Turkey.
Materials and Methods
Design. A cross-sectional, descriptive study was conducted at the Hospital of Medical Faculty, Atatürk University, Erzurum, Turkey. This 1,250-bed facility is an acute care, treatment, and health research center that comprises 21 units – 10 medical and 11 surgical service units (three intensive care units under the surgical service were included in this study). Data were collected on May 3, 2004 by the authors and three nurse assistants (research assistants and graduate students in the School of Nursing). The authors provided a 2-hour session to train the data collectors in the use of the BS and the NPUAP staging system. In this hospital, physicians evaluate and record pressure ulcers using NPUAP classification but only Stage III and Stage IV pressure ulcers are documented and Stage I and Stage II ulcers are not accurately reported. Therefore, every patient in the units was examined by the researchers and nurse assistants and assessments were noted on an observation form, yielding valid data.
Sample. The study population included all patients admitted to the medical, surgical, and intensive care units up to 24 hours before the study day (n = 382). Patients were eligible to participate if they met the following criteria: 18 years of age or older, able to speak Turkish, and a hospital stay of at least 24 hours before the assessment date.
Ethical approach. Approval to conduct this study was obtained from the appropriate authorities at each unit in the hospital and from the hospital administration. Patient participation was voluntary and verbal consent was obtained after patients received an explanation of the study. Family members provided informed consent for patients unable to communicate (eg, comatose patients).
Materials. Data were collected using a questionnaire, by skin inspection, and by assessing pressure ulcer risk using the BS.9 Information regarding other clinical characteristics such as age, hospital admission date, history of surgery, weight and height, albumin level, comorbidity or additional problems, and level of consciousness was obtained from the patients’ hospital records.
The questionnaire was designed by the researchers and included questions about the facility as well as patient demographic characteristics. Information obtained included patient age, gender, hospital admission date, serum albumin level, whether the patient underwent a surgical procedure under general anesthesia, level of consciousness, weight, height, presence of comorbid conditions and medical problems (eg, diabetes mellitus, hypertension, respiratory disease, vascular disease), length of hospital stay, and pressure ulcer locations. Level of consciousness was determined using the Glasgow Coma Scale20 and recorded in the patient record. Weight and height were used to calculate BMI and patients were classified as underweight (BMI: <18.5), normal weight (BMI 18.5 to 24.9), overweight (25.0 to 29.9), or obese (BMI >30).21 The NPUAP6 criteria were used to stage existing pressure ulcers.
The BS to determine pressure ulcer risk, created by Braden and Bergstrom in 1987, has been translated into many languages and is used on every continent.9 It includes six subscales: sensory perception, moisture, activity, mobility, nutrition, and friction/shear. These categories address the two primary etiologic factors of pressure ulcer development: intensity and duration of pressure and tissue tolerance for pressure. Reduced sensory perception, mobility, and activity may predispose a patient to intense and prolonged pressure, while the presence of moisture and friction/shear and less-than-optimal nutrition may alter tissue tolerance for pressure. Each subscale is ranked with a numerical score and scoring descriptions of all variables are provided. Five of the subscale scores (sensory perception, mobility, activity, moisture, and nutrition) range from 1 to 4; the sixth score, friction/shear, ranges from 1 to 3. The final BS score (range 6 to 23) is obtained by adding the six subscale scores. The lower the numeric scale score, the higher the patient’s predicted risk of developing a pressure ulcer. Incremental changes in the score indicate the level of risk as follows: 19 to 23, no risk; 15 to 18, at risk; 13 to 14, moderate risk; 10 to 12, high risk; 9 or below very high risk. The authors of the BS emphasize that continued testing has resulted in a recommendation that a cut-off score of 18 be used in clinical practice for many patient populations.9
Oˇguz and Olgun22 performed a methodological study on 30 patients admitted to the neurological clinic of a state hospital. Their study addressed the reliability and validity of utilizing the BS for assessments of pressure ulcers in a Turkish population. They reported a 0.95 coefficient for all scale items and coefficient values for subscales (sensory reception r = 0.77, moisture r = 0. 55, activity r = 0.79, mobility r = 0.78, nutrition r = 0.82, friction/shear r = 0.62).22 In a descriptive study of 45 bed-bound patients, Pinar and Oˇguz23 found that coefficient reliability of the BS was 0.95 for total items; values for subscales varied from 0.40 to 0.79. The validity and reliability of the Norton and the Braden risk evaluation scales also were compared; they were found to be high for both scales (r = 0.95, P <0.001). Analyses of the studies related to the Turkish version of the BS included internal consistency, reliability, and factor analysis. The authors translated and adapted the BS into Turkish and tested it for validity and reliability22,23; consequently, the scale has an acceptable internal reliability for the Turkish population and has been used in various patient populations in Turkey.23,24
The authors of the current study utilized a score of 18 to designate patients at risk for pressure ulcers. For purposes of this report, Chronbach’s alpha coefficient for overall scale items was 0.83 and alpha coefficient values for subscales varied from r = 0.85 to r = 0.75 (sensory reception r = 0.80, moisture r = 0.85, activity r = 0.75, mobility r = 0.75, nutrition r = 0.81, friction/shear r = 0.79); thus, the BS had an acceptable internal reliability for this study.
Statistical analysis. Independent variables included demographic and clinical characteristics and dependent variables included mean BS scores. Data were analyzed using SPSS® version 9.05 (Chicago, Ill) for Windows®. Descriptive statistics were utilized in order to examine the distributions and mean scores of the scale, subscales, and demographic data of patients. A chi-square test was used for comparisons between patients with and without pressure ulcers and between populations at risk and not at risk for pressure ulcer development, except for mean age and albumin level, which were assessed with a Student’s t-test. Pearson correlation analysis was performed to evaluate the correlation between pressure ulcer risk and length of hospital stay. Chronbach’s alpha coefficient was used to ensure adequate internal reliability of the BS. Differences were considered significant at P <0.05.
Results
On the day of the study, 382 eligible patients were on the units. Of those, 344 were included in study (90.1% participation rate) – 38 eligible patients (9.9%) were not included in the study; of these, 12 (31.6%) refused to participate in the study and 26 (68.4%) were not available for skin inspection in the ward.
Pressure ulcer prevalence. Forty (40) patients with pressure ulcers were identified for a prevalence rate of 11.6%. Of those pressure ulcers, 34 (9.9%) were hospital-acquired, implying a pre-hospitalization prevalence rate of 1.7% (n = six patients) (see Table 1).
 Patient characteristics. Of the 344 participants, 55.5% were men, 92.7% were conscious, and the mean age was 51.73 (SD 16.44; range 18 to 88) years. The men had statistically significant lower admission scores on the BS; also, more than half of patients with additional problems or comorbidities were male (56.9%, 95 of 167 patients). No significant differences were found regarding age, gender, or BMI among patients who did and those who did not have a pressure ulcer. However, differences were found between patient groups regarding the following variables: hospital unit of admission, surgical status, level of consciousness, presence of comorbidity or other medical problems, level of pressure ulcer risk, and serum albumin level. A more detailed analysis using chi-square or t tests demonstrated that a significantly greater proportion of patients with pressure ulcers were in the ICU (X2 = 42.56, df = 2, P = 0.000), comatose (X2 = 80.35, df = 2, P = 0.000), post surgery (X2 = 17.22, df = 1, P = 0.000), had a comorbidity or other medical problem (X2= 27.49, df = 1, P = 0.000), had a low albumin level (t = -2.78, mean 3.30±.64, P = 0.006), or were known to be at risk according to their BS scores (X2= 69.02, df = 1, P = 0.000). Most patients with a pressure ulcer also had comorbidities or additional medical problems (35 out of 40), including fracture (nine patients), diabetes mellitus (five), paralysis (five), urinary incontinence (four), heart failure (four), and other problems (eight).
Patient characteristics. Of the 344 participants, 55.5% were men, 92.7% were conscious, and the mean age was 51.73 (SD 16.44; range 18 to 88) years. The men had statistically significant lower admission scores on the BS; also, more than half of patients with additional problems or comorbidities were male (56.9%, 95 of 167 patients). No significant differences were found regarding age, gender, or BMI among patients who did and those who did not have a pressure ulcer. However, differences were found between patient groups regarding the following variables: hospital unit of admission, surgical status, level of consciousness, presence of comorbidity or other medical problems, level of pressure ulcer risk, and serum albumin level. A more detailed analysis using chi-square or t tests demonstrated that a significantly greater proportion of patients with pressure ulcers were in the ICU (X2 = 42.56, df = 2, P = 0.000), comatose (X2 = 80.35, df = 2, P = 0.000), post surgery (X2 = 17.22, df = 1, P = 0.000), had a comorbidity or other medical problem (X2= 27.49, df = 1, P = 0.000), had a low albumin level (t = -2.78, mean 3.30±.64, P = 0.006), or were known to be at risk according to their BS scores (X2= 69.02, df = 1, P = 0.000). Most patients with a pressure ulcer also had comorbidities or additional medical problems (35 out of 40), including fracture (nine patients), diabetes mellitus (five), paralysis (five), urinary incontinence (four), heart failure (four), and other problems (eight).
Most patients (28) had one ulcer; nine (22.5%) had two ulcers, and three (7.7%) had three ulcers for a total of 47 ulcers. The pressure ulcers observed most commonly were Stage I (34 ulcers, 72.3%), followed by Stage II (seven, 14.9%), Stage III (four, 8.5%), and Stage IV (two, 4.3%).
 Pressure ulcer location. Of the 47 pressure ulcers noted in 40 patients, the most common locations were the sacrum (38.3%), ischium (25.6%), and heel (23.4%) (see Table 2).
Pressure ulcer location. Of the 47 pressure ulcers noted in 40 patients, the most common locations were the sacrum (38.3%), ischium (25.6%), and heel (23.4%) (see Table 2).
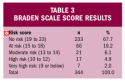
Braden Scale score results. Of the 344 patients assessed, 111 (32.3%) were found to be at risk for pressure ulcer development, with a BS score of 18 or lower (mean 14.78±2.66 in the at-risk group) (see Table 3).
Mean hospital length of stay before the day of the assessment was 11.31 days (SD 8.86; range 2 to 61 days, median = 9.00). For 43.3% of the patients, the length of stay was between 1 and 7 days.
 A statistically significant (negative) relationship was found between BS score results and hospital length of stay (r = 0-.159, P = .003). In other words, patient mean BS score decreased as hospital length of stay increased. Based on the BS score, patients found to be at greater risk for pressure ulcer development were older, male, underweight, residing in the ICU, postsurgical, or unconscious. Patients with stupor, comorbidities or medical problems, low albumin levels, and/or lower BS scores were also at greater risk for pressure ulcer development (see Table 4). While 111 patients were found to be at risk for pressure ulcer development according to a BS cut-off score of 18, 36 (32.4%) had and 75 (67.6%) had not developed a pressure ulcer. Although the BS score did not remain predictive in the regression analysis, the bivariate and descriptive statistical findings (eg, cross-tabs) support further exploration.
A statistically significant (negative) relationship was found between BS score results and hospital length of stay (r = 0-.159, P = .003). In other words, patient mean BS score decreased as hospital length of stay increased. Based on the BS score, patients found to be at greater risk for pressure ulcer development were older, male, underweight, residing in the ICU, postsurgical, or unconscious. Patients with stupor, comorbidities or medical problems, low albumin levels, and/or lower BS scores were also at greater risk for pressure ulcer development (see Table 4). While 111 patients were found to be at risk for pressure ulcer development according to a BS cut-off score of 18, 36 (32.4%) had and 75 (67.6%) had not developed a pressure ulcer. Although the BS score did not remain predictive in the regression analysis, the bivariate and descriptive statistical findings (eg, cross-tabs) support further exploration. 
Discussion
This study investigated the prevalence of patients with or at risk for pressure ulcers in a university hospital setting in Turkey as well as related clinical and patient characteristics. The prevalence rate for patients presenting with pressure ulcers was 11.6% on the day of the study, which is similar to previously published prevalence rates among other patient populations in acute care settings or hospitals.2,8,10,12 Study results also confirm that pressure ulcer rates are higher in surgical and comatose patients and in patients with a low albumin level and other diseases or medical problems.7,12,15,19 Ulcer locations most frequently noted were the sacrum, followed by ischium and heels; most ulcers were Stage I (72.3%). These results are consistent with those of a descriptive, cross-sectional study involving 922 patients conducted by Hug et al.12 They reported a Stage I pressure ulcer occurrence of 69%. Although most patients (28, 70%) had only one ulcer, a few had more than two ulcers (three, 7.7%). Previous studies demonstrated that the most common location for pressure ulcers are the sacrum or coccyx and the heels and that Stage I pressure ulcers are the most prevalent.1,11,12,25,26 In a recent study of surgical patients, Lindgren et al19 found the most common locations for pressure ulcers were the sacrum (29.8%), heels (19.3%), and ischial tuberosities (14%); Stage I was most common. Thus, because studies continue to reveal that pressure ulcers most commonly occur in locations subject to increased pressure from body weight on bony prominences (eg, sacrum, heels, hips, ankles, elbows, and occiput), initial skin assessment should include bony prominences to identify early signs of pressure damage.27
In this study, 32.3% of patients were at risk for pressure ulcer development (utilizing a BS cut-off of 18 or below). Lahmann et al2 reported that the proportion of all patients and residents at risk in hospitals and nursing homes was 29.0%, using the same BS cut-off score of 18 points, a number commonly used in clinical practice to assess patient risk and the need to plan preventive interventions.9 Utilizing the BS score to standardize risk assessment across populations in all types of institutions may have a large impact on the measured prevalence rate of at-risk patients noted by researchers.2 However, Ayello and Braden9 state that although sensitivity and specificity varied slightly among studies, collective evidence indicates that a cut-off score of 18 for the elderly and all ethnic groups is appropriate across all settings.
Patients in the current study found to be most frequently at risk for pressure ulcer development were male, older, unconscious, and postsurgical; patients with stupor, a low BMI and serum albumin, and other health problems known to be associated with pressure ulcer formation (eg, fracture, paralyses, diabetes, and incontinence) also were at risk. These findings confirm results of previous studies that examined risk factors associated with pressure ulcer development12,17 and other literature regarding pressure ulcers.5,7,14
In addition, bivariate analysis demonstrated a small, but statistically significant negative relationship between BS scores and length of hospital stay. The negative Pearson correlation for the mean total BS score and the mean length of stay in the hospital indicates that patient risk for pressure ulcers increased as the length of stay in the hospital increased (patient mean total BS scores decreased as length of stay in hospital increased). These results are in agreement with the previously published results of Hug at al.12
Also, the mean age of patients at risk for pressure ulcers was 56.12 years (SD 17.54), a result that corresponds with the reported results of other studies.10,12,15,26 In this study, no difference between men and women with respect to the prevalence of pressure ulcers was found, but more men than women had a low BS score. In addition, men had statistically significant lower admission scores on the BS and more than half of patients with additional problems or comorbidities were male (56.9%, 95 of 167 patients). These results suggest that male patients should be assessed for pressure ulcer risk at the time of admission and interventions should be initiated to prevent pressure ulcer development as quickly as possible during the hospital stay.
As expected, pressure ulcer prevalence was higher in ICU patients than in medical and surgical units and patient BS scores were lower. A majority of the patients in intensive care was assessed to be at risk and more than two thirds of these patients had a pressure ulcer on examination. Most patients with pressure ulcers (35, 87.5%) had comorbidities or additional medical problems such as (in descending order of frequency) hip fracture, paralysis, or diabetes mellitus, a finding that agrees with previously published studies involving medical and surgical patients.15 Lahmann et al2 indicated that geriatric wards and ICUs had the highest percentage of patients at risk for pressure ulcers and consequently higher rates of prevalence; whereas, Gunningberg11 reported the highest prevalence rate (59.3%) in geriatric care, followed by acute care departments including orthopedics (34.7%), internal medicine (23.6%), and surgery (21.8%). Comorbidity of any one disease increases patient risk for pressure ulcer development; risk also is increased in the elderly, particularly immobile patients – eg, patients with hip fractures constitute a group at high risk for developing pressure ulcers.11
In this study, pressure ulcer prevalence in surgical patients was higher and their mean BS scores lower than non-surgical patients, suggesting that most patients undergoing surgery are at risk for pressure ulcer development. This study did not analyze the patients’ condition during surgery or the type of surgery performed. However, the literature contains results suggesting a pressure ulcer prevalence rate range of 3.5% to 29.5 % in surgical patients.15 Patients in the preoperative, perioperative, and postoperative period are at risk for pressure ulcers due to immobility, possible comorbid circulation disorders, or other problems that increase risk of pressure ulcer development. Preoperative factors affecting the development of pressure ulcers in surgical patients include low preoperative hemoglobin, comorbidity of other disease processes, and a preoperative serum albumin level <3g/dL.7 Thus, it is commonly recognized that assessment, prevention, and early detection will help reduce the development of pressure ulcers in surgical patients at risk.
In the present study, pressure ulcer prevalence was higher in patients with lower serum albumin (<3.5 g/dL) and mean BS scores. In addition, underweight patients (utilizing BMI scores as a parameter for weight – a BMI cut-off <18.5) were at increased risk for pressure ulcer development. A direct causal relationship between nutrition and pressure ulcer development often is assumed, possibly due to the fact that impaired nutrition may influence tissue vulnerability to extrinsic factors such as pressure.27 In a recent literature review, Thomas28 explained that in some randomized, controlled clinical trials and prospective studies where decreased serum albumin was associated with pressure ulcers, the decrease may reflect the presence of inflammatory cytokine production rather than overall nutritional status. The European Pressure Ulcer Advisory Panel (EPUAP)27 recommends that, at a minimum, assessment of nutritional status should include regular weight checks, skin assessment, and documentation of food and fluid intake.
Pressure ulcer prevention must begin with identifying the patients at risk. Thus, patients should be assessed for pressure ulcer risk on admission and at periodic intervals thereafter during the hospitalization. Risk assessment should utilize a validated assessment tool such as the BS. The EPUAP29 suggests that a complete risk assessment should include general medical condition, skin assessment including moistness of the skin, presence or absence of incontinence, overall nutritional status, mobility, and pain level. The Panel also suggests daily assessment and documentation of skin condition.
Pressure ulcers were most prevalent in comatose patients, followed by surgical patients who had comorbidities (at least one disease) or other medical problems and low albumin level.
Study Limitations
Because the study focused on one large hospital, generalizability of the results is limited to similar surgical and medical settings. Further attention must be given to explicit definitions of the variables (eg, pressure ulcer size, treatment, and care or prevention interventions), permitting more comprehensive measurement related to patient risk for pressure ulcer development.
Conclusion
This study analyzed underlying causes of and risk factors for pressure ulcer development. Information regarding pressure ulcer development and prevalence can help clinicians take appropriate preventive measures. Results of this study may be useful for ongoing data comparison, enabling clinicians to increase their knowledge base in terms of pressure ulcer risk factor assessment and to revise nursing care plans accordingly. It should be noted that assessment for pressure ulcers in hospitalized patients is only a first step to prevent pressure ulcer development.
Findings described here suggest that prevention and assessment of pressure ulcers occurring in hospitals should be improved; thereby, possibly decreasing their prevalence. If healthcare institutions repeat these studies periodically and compare the results, changes in quality can be monitored and attention can be focused on further quality improvement. Based on this study, standardized risk assessment tools such as the BS should be used to determine the risk of pressure ulcer development and subsequent preventive measures undertaken to avert or at least decrease the rate of pressure ulcers in hospitalized patients.
Specifically, activities need to be implemented to lower prevalence in Turkey. In the Turkish healthcare setting, information relating to pressure ulcer prevention, prediction, and management is limited. The National Agency for Health Care Policy and Research guidelines on prevention and management of pressure ulcers have been shown to have specific relevance to Turkish hospitals. Experienced clinicians and researchers in the field of pressure ulcers must develop pressure ulcer prevention and treatment protocols. Also, an economic analysis to determine the expected cost saving associated with clinically effective pressure ulcer prevention and treatment protocols would be useful. Education is warranted; clinicians must be informed regarding the use of daily risk assessment scales to implement skin care interventions from the time the patient is admitted through the entire length of the hospital stay.
This study can serve as a baseline for developing a quality system for pressure ulcer prevention. Quality improvement activities may be initiated as a result of this study. A future repeat prevalence study will help determine the effectiveness of any new strategies of care that are implemented.
1. Hopkins B, Hanlon M, Yauk S, et al. Reducing nosocomial pressure ulcers in an acute care facility. J Nurs Care Qual. 2000;14(3):28-36.
2. Lahmann NA, Halfens RJG, Dassen T. Prevalence of pressure ulcers in Germany. J Clin Nurs. 2005;14(2):165-172.
3. Beckrich K, Aronovitch SA. Hospital-acquired pressure ulcers: a comparison of costs in medical vs. surgical patients. Nurs Econ. 1999;17(5):263-271.
4. Halfens RJG, Haalboom JRE. A historical overview of pressure ulcer literature of the past 35 years. Ostomy Wound Manage. 2001;47(11):36-43.
5. Nicol NH, Black JM. Management of clients with integumentary disorders. In: Black JM, Hawks JH, Keene AM (eds). Medical-Surgical Nursing: Clinical Management for Positive Outcomes. Volume 2, 6th ed. Philadelphia, Pa: WB Saunders Company; 2001:1295-1302.
6. National Pressure Ulcer Advisory Panel. NPUAP Staging Report 1998. Available at: http://www.npuap.org/positn6.html. Accessed April 3, 2004.
7. Armstrong D, Bortz P. An integrative review of pressure relief in surgical patients. AORN J. 2001;73(3):645-666.
8. Barzack CA, Barnett RL, Childs EJ, Bosley LM. Fourth national pressure ulcer prevalence survey. Adv Wound Care. 1997;10(4):18-26.
9. Ayello EA, Braden B. How and why to do pressure ulcer risk assessment. Adv Skin Wound Care. 2002;15(3):125-131.
10. Thoroddsen A. Pressure sore prevalence: a national survey. J Clin Nurs.1999;8(2):170-179.
11. Gunningberg L. Are patients with or at risk of pressure ulcers allocated appropriate prevention measures? Int J Nurs Pract. 2005;11(2):58-67.
12. Hug AKME, Ünalan H, Karamehmetoglu SS, Tüzün S Gürgöze M, Tüzün F. Bir egitim hastanesinde basi yarasi prevalansi ve basi yarasi gelisiminde etkili risk faktörleri (Pressure ulcer prevalence in a teaching hospital and risk factors associated with pressure ulcer development). Türkiye Fiziksel Tip ve Rehabilitasyon Dergisi (Turkish). 2001;47(6):1-10.
13. Lepistö M, Eriksson E, Hietanen H, Asko-Seljavaara S. Patients with pressure ulcer in Finnish hospitals. Int J Nurs Pract. 2001;7(4):280-287.
14. Nicol NH, Ruszkowski AM. Integumentary problems. In: Lewis SM, Heitkemper MM, Dirksen SR (eds). Medical-Surgical Nursing: Assessment and Management of Clinical Problems. Volume 1, 5th ed. London, UK: Mosby; 2003:516-521.
15. Schultz A, Bien M, Dumond K, Brown K, Myers A. Etiology and incidence of pressure ulcers in surgical patients. AORN J. 1990;70(3):434-449.
16. Spector WD, Fortinsky RH. Pressure ulcer prevalence in an Ohio nursing home: clinical and facility correlates. J Aging Health. 1998;10(1):62-80.
17. Vap PW, Dunaye T. Pressure ulcer risk assessment in long-term care nursing. J Gerontol Nurs. 2000;26:37-45.
18. Rosenberg CJ. New checklist for pressure ulcer prevention. J Gerontol Nurs. 2002;28(8):7-12.
19. Lindgren M, Unosson M, Krantz AM, Ek AC. Pressure ulcer risk factors in patients undergoing surgery. J Adv Nurs. 2005;50(6):605-612.
20. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. Lancet. 1974; 81-84.
21. Centers for Diseases Control and Prevention: BMI – Body Mass Index for Adults. Available at: http://www.cdc.gov/nccdphp/dnpa/bmi/bmi-adult.htm. Accessed January 16, 2004.
22. Oˇguz S, Olgun N. Basi yaralarinda Braden Ölçeginin kullanimi (Using Braden scale in pressure sores). In: Uluslar aras1 Katilimli V. Ulusal Hemsirelik Kongresi 1997. Kongre Kitab1 (Congress Book, Turkish), Izmir, DEÜ Rektörlü&euroü Matbaasi; 1998:71-75.
23. P1nar R, Oˇguz S. Norton ve Braden basi yarasi degerlendirme ölçeklerinin yataga bagimli ayni hasta grubunda güvenirlik ve geçerli€inin sinanmasi (Testing of reliability and validity of Norton and Braden pressure sore evaluation scales in the confined to bed patient group). In: Uluslar arasi Kat1imli VI. Ulusal Hemsirelik Kongresi 1998. Kongre Kitabi (Congress Book, Turkish). Ankara, Turkey: Damla Matbaacilik;1998:172-175.
24. Kurtulus Z, P1nar R. Braden skalasi ile belirlenen yüksek riskli hasta grubunda albümin düzeyleri ile basi yaralari arasindaki iliski (Relation between albumin levels and pressure sore in high-risk patients defined with Braden’s Risk Assessment tool). CÜ Hemsirelik Yüksekokulu Dergisi (CU J School Nurs, Turkish): 2003;7(2):1-10.
25. Carlson EV, Kemp MG, Shott S. Predicting the risk of pressure ulcers in critically ill patients. Am J Crit Care. 1999;8:262-269.
26. Pearson A, Francis K, Hodgkinson B, Curry G. Prevalence and treatment of pressure ulcers in Northern New South Wales. Aust J Rural Health. 2000;8(2):103-110.
27. European Pressure Ulcer Advisory Panel (EPUAP-2005). Nutritional guidelines for pressure ulcer prevention and treatment. Available at: http://www.epuap.org/glprevention.html. Accessed January 2, 2006.
28. Thomas DR. Issues and dilemmas in the prevention and treatment of pressure ulcers: a review. J Gerontol Med Sci. 2001; 56(6 suppl A):M328-M340.
29. European Pressure Ulcer Advisory Panel (EPUAP-2004) Pressure ulcer prevention guidelines. Available at: http://www.epuap.org/glprevention.html. Accessed June 8, 2006.













