A Post-hoc Analysis of Reduction in Diabetic Foot Ulcer Size at 4 Weeks as a Predictor of Healing by 12 Weeks
Abstract: Percent area reduction (PAR) after 4 weeks of diabetic foot ulcer (DFU) treatment has been suggested as a clinical monitoring parameter to distinguish DFUs that will heal within 12 weeks from those that will not despite standard wound care. The purpose of this post-hoc analysis of control DFU treatment outcomes from two published, randomized, controlled studies was to assess the relationship between PAR during early standard wound care and ulcer closure by week 12.
The proportion of DFUs healed after 12 weeks was 57% (39 out of 69; 95% confidence interval [CI], 44% to 68%) in study A and 52% (38 out of 73; 95% CI, 40% to 64%) in study B for wounds with ≥50% PAR by week 4 and 5% (three out of 64; 95% CI, 1% to 13%) and 2% (one out of 44; 95% CI, 0.1% to 12%), respectively, for DFUs with <50% PAR at week 4. Regardless of baseline size category, DFUs with <50% PAR at 4 weeks were less likely to heal by 12 weeks than DFUs with ≥50% PAR (P ≤ 0.001). Using pooled data, PAR at weeks 1 to 3 also varied between ulcers that did and did not heal after 12 weeks but sensitivity and specificity was highest on week 4. These findings confirm that percent reduction in wound size is an early predictor of treatment outcome and that protocols of care should be re-evaluated if ≥50% PAR is not achieved. Studies to assess DFU healing before and after 4 weeks of standard wound care are needed to further refine these guidelines of care.
Potential Conflicts of Interest: Dr. Snyder, Dr. Dauphinée, and Dr. Stavosky disclose they have received monetary compensation as consultants for Advanced BioHealing, Inc. Mr. Cardinal is an employee of Advanced BioHealing, Inc.
Please address correspondence to: Robert J. Snyder, DPM, CWS, Wound Healing Center, University Hospital and Medical Center, 7301 N. University Drive, Suite 305, Tamarac, FL 33321; email: drwound@aol.com. Financial support for data analysis and manuscript development was provided by Advanced BioHealing, Inc., La Jolla, CA.
Diabetic foot ulcers (DFUs) are among the most common complications of diabetes mellitus, with an annual incidence of 1% to 4% and lifetime risk of 15% to 25%.1-5 The morbidity associated with DFUs is high — >85% of lower-extremity amputations are precipitated by a DFU6,7 and approximately 15% of DFUs result in lower-extremity amputation.4,8 Delayed healing of DFUs can decrease patient mobility, reduce quality of life,9 and increase the risk of amputation.6 A meta-analysis10 of the control groups from nine DFU treatment studies showed that with standard good wound care 24% of ulcers heal after 12 weeks of care and 31% after 20 weeks of care. These results illustrate that even with good standard wound care DFUs remain difficult to heal. Prognostic indicators of wound closure can alert clinicians to reassess the therapeutic approach and consider more advanced interventions if necessary. In a retrospective analyses of 27,630 patients from a group of more than 150 wound care clinics in the US, investigators found negative prognostic factors for neuropathic DFU healing include baseline ulcer area >2 cm2, ulcer duration >2 months, and ulcer grade ≥3 (on a 6-point scale).11
In 2003, Sheehan et al,12 studying healing rates of DFU, confirmed what had previously been observed in other chronic wounds: percent area reduction (PAR) after 4 weeks of treatment is a robust predictor of healing.The purpose of this retrospective, secondary analysis from two previously published studies13,14 was to assess whether a PAR of 50% after 1 to 4 weeks of standard wound care is associated with DFU closure by 12 weeks in both small and larger wounds.
Methods and Procedures
Data were extracted from the original data sets of two previously published, randomized, controlled trials of a human fibroblast-derived dermal substitute for treating DFUs (ie, study A13 and study B,14 respectively) and included the control group subjects who completed each of these studies (N = 133 and 117, respectively). (Note: For study B, efficacy data was reported for ulcers with >6 weeks duration [n = 115]. For the purpose of the current analysis, all control subjects with evaluable data from study B were included [n=117] for final analysis). Raw data were provided by Advanced BioHealing Inc. (La Jolla, CA) and re-analyzed. Study data was extracted from two software databases containing patient identification numbers and wound planimetry values. Study A was conducted at 20 wound-care practice centers in the US and included adult persons with type 1 or type 2 diabetes and full-thickness DFUs affecting >1 cm2 of the plantar surface or heel that did not change appreciably and remained >1 cm2 during the 2 weeks of pre-trial standard care treatment. Study B was conducted at 35 wound-care practice centers in the US and included adults with type 1 or type 2 diabetes who had a DFU present for a minimum of 2 weeks before study participation. Study methods and results have previously been published.13,14
Patients were studied for at least 12 weeks and received standard wound care consisting of DFU debridement whenever clinically necessary, saline-moistened gauze dressings covered with dry gauze, adhesive fixation sheets, therapeutic footwear, and offloading instructions. Ulcer area was determined using similar methods in each trial; computer planimetry (determined from a bilayer acetate tracing) was used to measure ulcer area. Institutional review board approval/written informed consent was obtained from all patients in both studies.
Study Method and Data Analysis
Using the original study data (patient and ulcer descriptions and exact measurements gathered by the clinical investigators), PAR was calculated as the difference in ulcer area from baseline to time point, divided by the baseline, and multiplied by 100. Only control group patient data were analyzed. The primary variable assessed for this analysis was the derived PAR from weekly ulcer measurements from week 0 (baseline) through week 4.
 Data were dichotomized by PAR of <50% or ≥ 50% by week 4 to assess the association of PAR with DFU closure by 12 weeks. The original study stratification scheme was used to categorize ulcer size by baseline median area (≤1.51 cm2 and >1.51 cm2 for study A; ≤1.31 cm2 and >1.31 cm2 for study B) and the association of 50% PAR at 4 weeks to ulcer closure was assessed for DFUs above and below the median baseline ulcer area. Data from studies A and B were pooled to produce receiver operating characteristic (ROC) curves for PAR values at weeks 1, 2, 3, and 4 to determine which week was the best indicator of ulcer closure at week 12.15 The number of DFUs healed by week 12, stratified by 50% PAR at weeks 1, 2, 3, and 4, used pooled data to further illustrate the weekly differences. Ulcers with 100% PAR or an area of 0 cm2 by weeks 12 were classified as healed. DFUs not at 100% PAR or with an area >0 cm2 at week 12 were classified as unhealed.
Data were dichotomized by PAR of <50% or ≥ 50% by week 4 to assess the association of PAR with DFU closure by 12 weeks. The original study stratification scheme was used to categorize ulcer size by baseline median area (≤1.51 cm2 and >1.51 cm2 for study A; ≤1.31 cm2 and >1.31 cm2 for study B) and the association of 50% PAR at 4 weeks to ulcer closure was assessed for DFUs above and below the median baseline ulcer area. Data from studies A and B were pooled to produce receiver operating characteristic (ROC) curves for PAR values at weeks 1, 2, 3, and 4 to determine which week was the best indicator of ulcer closure at week 12.15 The number of DFUs healed by week 12, stratified by 50% PAR at weeks 1, 2, 3, and 4, used pooled data to further illustrate the weekly differences. Ulcers with 100% PAR or an area of 0 cm2 by weeks 12 were classified as healed. DFUs not at 100% PAR or with an area >0 cm2 at week 12 were classified as unhealed.
Normally distributed continuous variables verified using Anderson-Darling techniques were summarized by means and standard deviations; 95% confidence intervals (CI) were calculated for proportions based on the binomial distribution (exact method). Non-normally distributed variables were summarized by medians and interquartile ranges. Associations between categorical variables and binary outcomes were analyzed using 2 × 2 chi-square tables. The Kruskal-Wallis test was used to assess the equality of medians from populations with non-normal distributions. Hypothesis testing was conducted at α = 0.05. Statistical tests and ROC curves were executed using Minitab (version 15.1.20.0; Minitab, Inc., State College, PA) and MATLAB (version 7.6.0 R2008a; The Mathworks, Natick, MA). 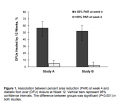
Results
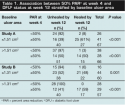
The median baseline ulcer area was 1.51 cm2 (range, 0.66 to 24.65 cm2; SD 3.45) in study A and 1.31 cm2 (range, 0.50 to 14.45 cm2; SD 2.14) in study B. Of the DFUs analyzed, 75% had a baseline ulcer area <3 cm2, which is consistent with those observed in clinical practice settings. In study A, 32% (42 out of 133) of wounds and in study B 33% (39 out of 117) of wounds were healed after 12 weeks. For DFUs with ≥50% PAR by week 4, 57% (39 out of 69; 95% CI, 44% to 68%) in study A and 52% (38 out of 73; 95% CI, 40% to 64%) in study B had healed by 12 weeks (see Figure 1). In contrast, 5% (three out of 64; 95% CI, 1% to 13%) and 2% (one out of 44; 95% CI, 0.1% to 12%) of DFU with <50% PAR at week 4 had healed by 12 weeks (study A and B, respectively).
Regardless of baseline size category, DFUs with <50% PAR at 4 weeks were less likely to heal by 12 weeks than DFUs with ≥50% PAR (P ≤ 0.001) (see Table 1). In addition, for DFUs that failed to heal by 12 weeks, PAR at 12 weeks was greater for wounds with ≥50% PAR than for wounds with <50% PAR at week 4 (84.9% and 42.4% study A and 78.1% and 32.2% study B, respectively; P ≤0.001).  DFUs that healed by week 12 had a significantly greater median PAR during weeks 1 to 4 (P <0.01 for all time points) compared with DFUs that did not heal (see Figure 2). Overall, the median (±SD) PAR at 4 weeks was 93.8% ± 17.4% and 38.9% ± 39.8%, respectively, for ulcers that healed and did not heal by 12 weeks.
DFUs that healed by week 12 had a significantly greater median PAR during weeks 1 to 4 (P <0.01 for all time points) compared with DFUs that did not heal (see Figure 2). Overall, the median (±SD) PAR at 4 weeks was 93.8% ± 17.4% and 38.9% ± 39.8%, respectively, for ulcers that healed and did not heal by 12 weeks.
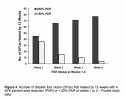
The sensitivity and specificity of the relationships between PAR values at weeks 1 to 4 and complete healing (100% PAR) by week 12 were plotted as ROC curves using combined data from both studies. The area under the ROC curve was higher on week 4 (.91) than on week 1, 2, or 3 (area under the ROC curve .71, .82, and .86, respectively) (see Figure 3). Using the pooled data, large differences were observed in the number of ulcers healed at 12 weeks for the ≥50% versus <50% PAR groups at weeks 1 through 3; however, the disparity is much greater by week 4 (see Figure 4).
Discussion
Standard wound care generally consists of appropriate sharp debridement of the ulcer, adequate offloading (total contact or removable cast), and moist gauze dressings. Complete wound closure is the ultimate goal in the treatment of a DFU and is associated with improved quality of life and patient mobility9,16 The results of this analysis found <33% of ulcers healed after 12 weeks of standard wound care. Clinical experience supports re-evaluating the treatment regimen if the ulcer is not progressively healing.2
 The results of this secondary analysis confirms the work of others12,17-19 that DFU wound area reduction at 4 weeks is a predictor of DFU healing at 12 weeks and that a change in treatment regimen is needed if an ulcer is not progressively healing. In a retrospective cohort study of more than 20,000 patients from a US-based wound care clinic system, Margolis et al17 found that using percent change in wound size by week 4 accurately discriminated DFUs that would heal from those that would not heal by week 20 approximately 69% of the time.
The results of this secondary analysis confirms the work of others12,17-19 that DFU wound area reduction at 4 weeks is a predictor of DFU healing at 12 weeks and that a change in treatment regimen is needed if an ulcer is not progressively healing. In a retrospective cohort study of more than 20,000 patients from a US-based wound care clinic system, Margolis et al17 found that using percent change in wound size by week 4 accurately discriminated DFUs that would heal from those that would not heal by week 20 approximately 69% of the time.
Sheehan et al12 assessed the ability of the 4-week healing rate to predict complete healing over a 12-week period in a large prospective multicenter trial of 203 patients with DFUs. The midpoint between the PAR from baseline at 4 weeks in patients healed versus those not healed at 12 weeks was found to be 53%. Subjects with a PAR in ulcer size >53% in 4 weeks had a 12-week healing rate of 58%; whereas, persons with reduction in ulcer area <53% in 4 weeks had a healing rate of only 9% (P <0.01).
 A subanalysis of data from a randomized controlled trial (N = 162) by Lavery et al18 found a similar association between PAR at 4 weeks and complete healing after 16 weeks. Patients with large, chronic DFUs following partial foot amputation received standard moist wound therapy (n = 77) or negative-pressure wound therapy (n = 85). PAR at 4 weeks was predictive of DFU healing at 16 weeks (odds ratio 1.04; 95% credible set 1.02 to 1.05). Results indicate that for every additional PAR percentage point from baseline at 4 weeks, the likelihood of healing by 16 weeks increased by 4%. Ulcers that had at least 60% PAR by week 4 had a 77% probability of healing compared with 30% for DFUs with <60% PAR at week 4.
A subanalysis of data from a randomized controlled trial (N = 162) by Lavery et al18 found a similar association between PAR at 4 weeks and complete healing after 16 weeks. Patients with large, chronic DFUs following partial foot amputation received standard moist wound therapy (n = 77) or negative-pressure wound therapy (n = 85). PAR at 4 weeks was predictive of DFU healing at 16 weeks (odds ratio 1.04; 95% credible set 1.02 to 1.05). Results indicate that for every additional PAR percentage point from baseline at 4 weeks, the likelihood of healing by 16 weeks increased by 4%. Ulcers that had at least 60% PAR by week 4 had a 77% probability of healing compared with 30% for DFUs with <60% PAR at week 4.
Coerper et al19 prospectively studied 704 subjects with DFU at a single treatment center in Germany and found a PAR of 50% at week 4 to be predictive of wound healing at week 12. Of persons with ≥50% PAR at week 4, 52.3% healed by week 12 compared with 18.4% of persons with <50% PAR at week 4 (P = .0001). According to the current analyses, more than half of the DFUs that achieved ≥50% PAR at week 4 went on to heal by week 12. In addition, ulcers that were likely to heal by week 12 tended to have a rapid progression in PAR early in the treatment period (weeks 1 to 4). Results from the ROC suggest that PAR at week 4 (rather than week, 1, 2, or 3) is the best prognostic indicator of healing by 12 weeks because it provides the highest specificity and sensitivity.
A commonly cited predictor of DFU healing is baseline ulcer area.11 Although the effect of baseline area was not independently assessed in this analysis, a reduction of 50% wound area at 4 weeks was predictive of healing at 12 weeks independent of dichotomized initial wound size (smaller, ≤ the group median; larger, > the group median). This finding reinforces the importance of 50% PAR at 4 weeks for DFU assessment in patients receiving standard wound care, but should not be interpreted to mean that baseline DFU area is not a prognostic factor in healing. Appropriate wound care should be provided from the onset of the course of treatment to maximize clinical outcomes. The ability to utilize an interim time point to assess the efficacy of standard wound care provides the opportunity to improve outcomes by considering treatment modification.
Limitations
Potential study limitations with this analysis are associated with any post-hoc data analysis and include the reliability of the statistical significance of endpoints that are not the primary study endpoint. Ulcer duration >2 months is a known harbinger of poor ulcer healing.11 The average ulcer duration was 46.5 weeks in study A and 67 weeks in study B. Neither this variable nor the location of the ulcer was included in the analysis and ulcer area was stratified using the original cutoff values specified in the studies. Finally, PAR was calculated in the original studies using two-dimensional ulcer surface area (cm2) measurements gathered from acetate tracings, a method that may be subject to measurement error because it does not capture ulcer depth, which may be substantial in some cases.
Conclusion
This post-hoc analysis of data from clinical study participants whose DFU were managed with a commonly used wound care protocol indicates that <50% PAR after 4 weeks of treatment predicts DFU failure to heal by 12 weeks. This finding was observed in both small and larger (>1.5 cm2) ulcers. Data presented support the conclusions reached in previous trials. An ulcer is less likely to heal by 12 weeks if there is little reduction in wound area during the first 4 weeks of care. Therefore, 4 weeks may be used as a clinical decision point to re-evaluate current DFU management and if 50% PAR is not achieved, a change in patient protocol of care is warranted. Further analyses are needed to understand the healing process and changes in PAR affecting closure rates in DFUs before and after 4 weeks.
Acknowledgments
The authors thank Beatriz Manzor Mitrzyk, PharmD, BCPS, and Amy M. Horton, PharmD, CMPP, for their writing and editorial assistance with this manuscript.
1. Reiber GE, Ledoux WR. Epidemiology of diabetic foot ulcers and amputations. In: Williams R, Herman W, Kinmonth AL, Wareham NJ, eds. The Evidence Base for Diabetes Care. Hoboken, NJ: John Wiley & Sons; 2002:641–665.
2. Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice: neuropathic diabetic foot ulcers. N Engl J Med. 2004;351:48–55.
3. Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–1724.
4. Sanders LJ. Diabetes mellitus: prevention of amputation. J Am Podiatry Med Assoc. 1994;84:322–328.
5. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–228.
6. Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13:513–521.
7. Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes Metab Res Rev. 2000;16:S75–S83.
8. Ramsey SD, Newton K, Blough D, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22:382–387.
9. Goodridge D, Trepman E, Sloan J, et al. Quality of life of adults with unhealed and healed diabetic foot ulcers. Foot Ankle Int. 2006;27:274–280.
10. Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta-analysis. Diabetes Care. 1999;22:692–695.
11. Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: predicting which ones will not heal. Am J Med. 2003;115:627–631.
12. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in ulcer area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26:1879–1882.
13. Pollak RA, Edington H, Jensen JL, Kroeker RO, Gentzkow GD. A human dermal replacement for the treatment of diabetic foot ulcers. Wounds. 1997;9(1):175–183.
14. Marston WA, Hanft J, Norwood P, Pollack R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers. Diabetes Care. 2003;26:1701–1705.
15. Zweig M, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577.
16. American Diabetes Association. Consensus Development Conference on Diabetic Foot Wound Care. Boston, Massachusetts. April 7–8, 1999. Diabetes Care. 1999;22:1354–1360.
17. Margolis DJ, Gelfand JM, Hoffstad O, Berlin JA. Surrogate end points for the treatment of diabetic neuropathic foot ulcers. Diabetes Care. 2003;26:1696-1700.
18. Lavery LA, Barnes SA, Keith MS, Seaman JW, Armstrong DG. Prediction of healing for postoperative diabetic foot wounds based on early wound area progression. Diabetes Care. 2008;31:26–29.
19. Coerper S, Beckert S, Küper MA, Jekov M, Königsrainer A. Fifty percent area reduction after 4 weeks of treatment is a reliable indicator for healing — analysis of a single-center cohort of 704 diabetic patients. J Diabetes Complications. 2009;23:49–53.













