A Cross-sectional Validation Study of Using NERDS and STONEES to Assess Bacterial Burden
Abstract
All chronic wounds are colonized by micro-organisms. Although the presence of bacteria is not necessarily harmful, and may be beneficial in some instances, accurate evaluation of wound-related bacterial damage and infection is crucial. A cross-sectional validation study involving 112 patients was conducted to estimate the specificity and sensitivity of clinical assessment variables individually and in combination to determine the presence and quantity of bacteria in the wound.
The average age of study participants was 66 years (range 33 to 95 years) and most had leg (44) and foot (68) ulcers of approximately 6 months’ duration. Wounds were evaluated using a mnemonic developed to evaluate the presence or absence of clinical signs of critical colonization (NERDS©) or infection (STONEES©) and results compared to semi-quantitative swab cultures. Wounds with debris, increased exudate, and friable tissue were found to be five times more likely to have scant or light bacterial growth; whereas, wounds with elevated temperature were eight times more likely to have moderate or heavy bacterial growth. When combining any three clinical signs, the sensitivity was 73.3% for scant or light and 90% for moderate and heavy bacterial growth and the specificity was 80.5% and 69.4%, respectively. Considering the importance of this clinical diagnosis, studies to examine the predictive validity of these assessment variables and culture results are warranted.
Potential Conflicts of Interest: Dr. Woo and/or Dr. Sibbald is a consultant, investigator, and/or speaker for one or more of the following companies/organizations: 3M (St. Paul, MN), Coloplast Corp. (Minneapolis, MN), Mölnlycke Health Care (Norcross, GA), Covidien (Mansfield, MA), Gaymar (Orchard Park, NY), KCI (San Antonio, TX), Systagenix Wound Management (Quincy, MA), Tyco International Ltd. (Mansfield, MA), ConvaTec (Skillman, NJ), Registered Nurses Association of Ontario (Canada), and the Government of Ontario.
Please address correspondence to: Kevin Y. Woo, RN, MSc, PhD, ACNP, GNC(C), FAPWCA, Wound Healing Clinic, East Room 1016, Women’s College Hospital, 76 Grenville Street, Toronto, Ontario M5S 1B2 Canada; email: kevin.woo@wchospital.ca.
All chronic wounds are invariably colonized by a complex ecology of micro-organisms.1 More than 90% of chronic wounds have been found to host polymicrobial flora containing an average range of 1.6 to 4.4 bacterial species depending on the type of ulcer.2 The presence of nonreplicating micro-organisms on the wound surface that do not evoke clinical host response is referred to as contamination. As the micro-organisms continue to proliferate within the wound, critical colonization (ie, the presence of bacteria in the superficial wound compartment that are associated with a host inflammatory response that delays or prevents healing) and wound infection can occur, causing clinical host injury3 (see Table 1).
Although the presence of bacteria is not necessarily pathological and may actually facilitate normal healing,2 wound healing has been found to be noticeably compromised when the bacterial burden crosses a certain colonization threshold to overcome host resistance or incorporates more than four pathological bacterial species.4 Based on an analysis of wound fluid from their seminal case series, Bendy et al5 reported chronic pressure ulcer healing was significantly thwarted at a bacterial load of 1.0 x 106 or higher number of colony forming units per gram (CFU/g) of tissue.
 The mechanisms explaining how bacterial burden affects healing are multifaceted and complex. Bacteria compete for nutrients and oxygen that are essential for wound healing activities. Bacterial exotoxins and endotoxins are generated and diffused into the wound milieu, impairing normal cellular functions such as collagen deposition and cross-linking that potentially contribute to surgical wound dehiscence.2 Bacteria also produce proteases and stimulate activated leukocytes to generate similar enzymes such as matrix metalloproteases (MMPs).6 An influx of proteases and associated activities in chronic wounds have been linked to stalled healing in several prospective ex vivo studies,7-9 as the degradation of extracellular matrix and growth factors occurs more rapidly than their synthesis, hindering the wound from progressing toward the proliferative phase.10
The mechanisms explaining how bacterial burden affects healing are multifaceted and complex. Bacteria compete for nutrients and oxygen that are essential for wound healing activities. Bacterial exotoxins and endotoxins are generated and diffused into the wound milieu, impairing normal cellular functions such as collagen deposition and cross-linking that potentially contribute to surgical wound dehiscence.2 Bacteria also produce proteases and stimulate activated leukocytes to generate similar enzymes such as matrix metalloproteases (MMPs).6 An influx of proteases and associated activities in chronic wounds have been linked to stalled healing in several prospective ex vivo studies,7-9 as the degradation of extracellular matrix and growth factors occurs more rapidly than their synthesis, hindering the wound from progressing toward the proliferative phase.10
 In view of the ubiquitous presence of microbes, the clinician must discern whether bacterial balance (contamination or colonization) or bacterial damage (critical colonization or infection) has occurred in order to institute appropriate interventions.
In view of the ubiquitous presence of microbes, the clinician must discern whether bacterial balance (contamination or colonization) or bacterial damage (critical colonization or infection) has occurred in order to institute appropriate interventions.
The purpose of this cross-sectional validation study was to estimate the specificity and sensitivity of the NERDS©and STONEES© variables individually and in combination to determine wound infection and their relationship to the quantity of bacteria in the wound.
Literature Review
An accurate evaluation of wound-related bacterial damage is crucial but an ideal approach to determine the presence of superficial critical colonization or deep wound infection is not universally accepted. Routine wound culturing with a bacterial swab may capture only contaminants on the wound surface rather than the bacteria invading the deep or surrounding skin wound compartment.11,12 The concordance between superficial swabs and biopsies has been found to range between 52% and 83% in patients across different wound types.13,14 Even with these limitations, through an analysis of bacterial swab culture results of 60 diabetic foot ulcers in a cross-sectional study, Slater el al15 identified the entire spectrum of isolates from tissue specimens that did not extend to bone in 90% of diabetic foot wounds (Grade 1 and 2 according to the University of Texas Wound Classification System). Pellizzer et al16 followed 29 patients with diabetic foot ulcers for 30 days and reported that the number of isolates yielded from swabs and biopsies were similar (68 versus 60, respectively).
From a clinical perspective, the diagnosis of wound infection may be best confirmed and supported by clinical signs and symptoms. Cutting and Harding17 proposed that evidence of red friable tissue, exuberant granulation, increased discharge, and new devitalized tissue, along with other criteria (a total of 15 items), all related to a greater probability of chronic wound infection. Based on these criteria, Cutting18 compared clinical assessment of 40 wounds (excluding burns and leg ulcers) with bacterial swab results and nurses’ evaluation of the same wounds to ascertain infection status; in 39 out of 40 wounds (97.5%), the researcher’s assessment findings were consistent with heavy growth noted in bacteriology reports but agreement between nurses and the researcher was only 47.5% (38 out of 80 comparisons). On 19 occasions, nurses considered the presence of infection but the researcher refuted these conclusions, indicating inconsistent assessment and potentially wrong diagnosis.
Cutting and White19 later proposed distinct sets of criteria for infection based on wound etiology. However, this type of classification is difficult to apply to practice; the value is mainly heuristic, not pragmatic. In Gardner’s20 cross-sectional descriptive study, a checklist was formulated based on validated symptoms and signs of infection including pain, exudate, delayed healing, discoloration of granulation tissue, friable granulation tissue, pocketing at the base of the wound, foul odor, and wound breakdown. Quantitative biopsy cultures >106 CFU/g of wound tissue (equivalent to heavy bacterial growth) were used as the criteria to determine the infection status of each study wound. A large number of infected wounds exhibited delayed healing and the presence of friable granulation tissue, with sensitivity values of 0.81 and 0.82, respectively. Increasing pain, increased warmth (determined by touch), and foul odor were present in fewer than half of the infected wounds with sensitivity values of 0.36, 0.18, and 0.36, respectively. Therefore, no single sign is sensitive for the diagnosis of superficial increased bacterial burden or deep infection. Gardner20 also demonstrated that perception of increasing pain was not significantly different between patients with or without diabetes in the presence of wound infection.
In their comparative study, Bowler and Davies1 evaluated the microbiology of leg ulcers (n = 74) that were categorized as either infected or noninfected based on clinical assessment. Although the evaluative criteria were not specified, the authors reported that a significantly greater mean number of bacteria was found in infected than noninfected ulcers (P <0.05). In a large cohort study (n = 1,229),21 more than half (58%) of the patients with diabetes presenting with a new foot ulcer were diagnosed with infection based on two or more physical signs and symptoms: frank purulence, local warmth, erythema, lymphangiitis, edema, pain, fever, and foul smell.
None of the reviewed studies differentiated local superficial wound damage from deep or surrounding tissue infection based on observable signs. This distinction could provide timely guidance for point-of-service clinicians to decide whether topical or systemic antimicrobial agents should be selected. However, Serena et al22 cautioned about potential underdiagnosis of wound infection if the diagnosis is based solely on clinical assessment. According to their analysis of 352 patients with venous leg ulcers in a clinical trial, Serena et al22 found that 26% of the study ulcers lacked the usual visual clinical signs of infection but were deemed infected based on quantitative biopsy results. With high host resistance, even high numbers of bacteria may be unable to damage host tissue (no clinical infection or delayed healing).
Another issue is that the identified individual clinical signs used to determine infection are tenuous and lack standardized definition. Greenwald et al’s23 evaluative study of 115 wounds determined the overall agreement on the presence of wound infection between two trained assessors was not always consistent (kappa = .57). In preparing the current study, Woo and Sibbald surmise that the relatively low concordance rate may be explained by the lack of clear definitions of the classical signs, leading to differing perceptions and interpretation of infection status. Wilson et al24 prospectively evaluated 5,804 surgical wounds in 4,773 patients using four common definitions of surgical site infection: 1) the Centers for Disease Control’s (CDC) 1992 definition,25 2) the Nosocomial Infection National Surveillance Survey (NINSS) modification of the CDC definition,26 3) the presence of pus, and 4) the ASEPSIS wound assessment scoring method27 (ie, additional treatment, the presence of serous discharge, erythema, purulent exudate, separation of deep tissue, isolation of bacteria, and duration of inpatient stay). Although certain assessment parameters are common across the four definitions (eg, purulent exudate, erythema, and separation of deep tissue), determination of the presence of wound infection was variable. The mean percentage of wounds identified as infected differed substantially with the different definitions; 19.2% (95% confidence interval [CI] of 18.1% to 20.4%) using the CDC definition, 14.6% (95 % CI 13.6% to 15.6%) with the NINSS version, 12.3% (95% CI 11.4% to 13.2%) with pus alone, and 6.8% (95% CI 6.1% to 7.5%) with an ASEPSIS score >20. In another survey,28 six physicians were asked to determine if infection was present based on clinical photographs of wounds from 120 patients. Sensitivity for overall impression of infection ranged from 37% to 90%, indicating great variability.
Based on the existing literature and extensive clinical experience, Sibbald et al29 created an assessment model using the mnemonic NERDS (nonhealing, increased exudate, red friable granulation, debris, and smell) and STONEES (deep infection, increased size, increased temperature, Os [probes to bone], new areas of breakdown, edema/erythema, increased exudate, and smell) (see Table 1 and Table 2) to categorize two levels of bacterial damage: superficial critical colonization or deep 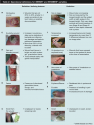 infection. Existing literature provided face validity for the model.
infection. Existing literature provided face validity for the model.
Materials and Methods
From June 2006 until November 2006, a total of 112 patients with leg and foot ulcers of various etiologies were recruited to participate in this prospective cross-sectional study. For patients with multiple ulcers, only one ulcer was selected for evaluation in the study. All patients were adults (at least 18 years old), fluent in English, and able to provide consent for participation in the study. The study was approved by the local research ethics board. Patients were enrolled from ambulatory wound care clinics and/or were home care clients. Each wound was evaluated once by an advanced practice nurse or a physician who assessed the wound for the presence or absence of clinical signs of superficial critical colonization or deep infection using the NERDS© and STONEES© variables (see Table 1 and Table 2 for operational definitions). Both assessors have extensive experience in chronic wound care and use of the assessment model.
Exudate levels were determined based on the amount of wound fluid staining that was left on the wound dressings. A change in exudate levels was documented as an increase in wound exudate with more than 50% of the dressing stained with exudate. After dressings were removed, wounds were evaluated for the presence or absence of debris, slough, and discolored granulation tissue on the wound base and the presence or absence of unpleasant or foul odor. Wounds were cleansed gently with saline-soaked gauze before wound margin edema and erythema (present or absent) were assessed. The presence of friable unhealthy granulation tissue that bleeds easily was assessed using a sterile instrument to gently probe the surface and wound base. Wound size was estimated by measuring the longest length and the widest width perpendicular to the length.30 Change in wound size at the time of the visit was recorded according to patient history or existing documentation. A sterile cotton applicator was used to determine wound depth and test whether the wound probed to bone, and a handheld infrared thermometer was used to compare the temperature of the periwound area with a similar location on the opposite extremity. The presence or absence of new areas of breakdown, including satellite lesions, was assessed and documented.
Microbiological analysis. To ensure that surface bacterial contaminants were not sampled, wounds were cleansed or irrigated with normal saline until all visible debris was washed away.14 This was followed by rotating the swab tip in a 1-cm2 area of the cleanest part of the wound, preferably in an area of granulation. Adequate pressure was used to extract tissue exudate for successful culturing. The swab then was rotated 360° and placed in the transport media (Levine technique14). The swabs were sent to a local laboratory to be processed in a timely fashion. To provide semi-quantitative culture data, the bacterial swabs were inoculated onto standard media in a Petri dish and serially diluted and streaked into four quadrants on the culture plate. Primary isolation plates were assessed after 5 days. Bacterial species isolated from the four quadrants were reported as scant (first quadrant), light (second quadrant), moderate (third quadrant), or heavy growth (fourth quadrant). Critical colonization (NERDS© variables) was evaluated by scant to light bacterial growth and STONEES© variables (deep wound infection) were evaluated by moderate to heavy growth. In a nonrandomized prospective study, Ratliff and Rodeheaver31 evaluated semi-quantitative swabs to determine bacterial burden (n = 124). Wounds where quantitative swabs revealed 105 or more bacteria/cm2 were defined as infected. Swabs that yielded moderate to heavy bacterial growth in quadrants 3 and 4 were correlated to wound infection (105 or more bacteria/cm2) with a sensitivity of 79%.
Statistical analysis. Each NERDS© and STONEES© variable was coded as a dichotomous variable based on whether the specific sign was present or absent on clinical evaluation. Data were entered into a computerized statistical program (SPSS version 16.0, Chicago, IL) and analyzed by the investigators. Odds ratios were calculated to determine the probability of bacterial growth and quantity in subjects who exhibited each individual sign and in combination of two to four signs. The accuracy of NERDS© and STONEES© to assess scant/light or moderate to heavy bacterial growth was estimated by computing the sensitivity and specificity for each clinical sign and combination of two to four signs.
Results
A total of 112 patients with 44 leg ulcers and 68 foot ulcers were evaluated in an ambulatory wound care clinic and on community visits. Most patients were male (60.4%); average age of all subjects was 66 years (range 33 to 95). The average duration of the ulcers was 6.3 months. Leg ulcers were related to venous disease (28 patients, 63.6%), lymphedema (six, 13.6%), arterial disease (two, 4.5%), mixed venous arterial disease (five, 11.3%), and miscellaneous etiologies (three, 6.8 %). The majority of patients with foot ulcers (67.6%) was diagnosed with diabetes mellitus — of these, 39.7% had neuropathy, 25% had a diagnosis of neuro-ischemic changes, and 2.9% did not have identified ischemia or neuropathy.
The odds ratios for individual NERDS© variables and scant/light bacterial growth ranged from 0.42 (CI = .18 to .97) to 5.63 (CI = 2.19 to 14.45) and from 2.76 (CI = 1.04 to 7.31) to 8.05 (CI = 2.90 to 22.38) for individual STONEES©  variables and moderate/heavy growth (see Table 3). Wounds were found to be five times more likely to have a scant/light growth of bacteria in the presence of debris, increased exudate, and friable tissue. Wounds with elevated temperature were eight times more likely to be associated with heavy bacteria growth.
variables and moderate/heavy growth (see Table 3). Wounds were found to be five times more likely to have a scant/light growth of bacteria in the presence of debris, increased exudate, and friable tissue. Wounds with elevated temperature were eight times more likely to be associated with heavy bacteria growth.
Sensitivity ranged from 32 for nonhealing wound status to 87 for edema. Clinical signs with the highest specificity were new breakdown, smell, and red friable tissue. By collating any three clinical signs exhibited in the assessed wounds, the sensitivity for NERDS© increased to 73.3% (specificity 80.5) and to 90% (specificity 69.4) for STONEES© (see Table 4).
Discussion
All chronic wounds contain bacteria on the surface and are considered contaminated by many external sources of micro-organisms from the environment — dressings, the patient’s skin and excrements, and the hands of patients and healthcare providers. When host resistance is compromised, micro-organisms can proliferate, which leads to wound surface critical colonization and deeper infection.2,29 Tissue damage inflicted by micro-organisms can de ter wound healing. Early recognition and management of infection is integral to best practices for chronic wound care. The study results suggest that the level of bacterial growth can be assessed by experienced clinicians using the clinical variables included in the mnemonics NERDS© (scant to light growth) and STONEES© (moderate to heavy growth). Moderate to heavy bacterial growth (STONEES©) indicated by semi-quantitative bacteriology report has been linked to clinical infection,31,32 requiring the use of systemic antimicrobial agents. Scant/light bacterial growth, on the other hand, has been proposed to be associated with critical colonization and may require topical antimicrobial treatment.33 The feasibility of using this approach to assess wound infection has been reported in two case studies.34,35
ter wound healing. Early recognition and management of infection is integral to best practices for chronic wound care. The study results suggest that the level of bacterial growth can be assessed by experienced clinicians using the clinical variables included in the mnemonics NERDS© (scant to light growth) and STONEES© (moderate to heavy growth). Moderate to heavy bacterial growth (STONEES©) indicated by semi-quantitative bacteriology report has been linked to clinical infection,31,32 requiring the use of systemic antimicrobial agents. Scant/light bacterial growth, on the other hand, has been proposed to be associated with critical colonization and may require topical antimicrobial treatment.33 The feasibility of using this approach to assess wound infection has been reported in two case studies.34,35
The individual signs of NERDS© and STONEES© each have a clear definition of the clustered signs and a rational for inclusion in the mnemonic. In this study, the odds ratio for nonhealing status, defined as wounds that were not 20% to 40% smaller in 4 weeks, was relatively low compared to other items. This may be the result of the study design because wounds were assessed only once and the response was based on patients’ recall ability and subjective impression of whether the wound was healing instead of actual wound measurements over time. Previous clinical studies have shown that increased bacterial burden is associated with high exudate levels.18,36,37 Although moisture balance is essential to all phases of healing,38 excess moisture can be deleterious.39 The validity of exudate as an indicator of increased bacterial burden in chronic wounds was substantiated by the calculated odds ratio (5.36, CI = .54 to 53.66 for superficial bacterial damage and 4.13, CI = 1.72 to 9.91 for deep infection). In fact, 70% of the wounds with increased exudate in the current study were correctly identified as infected, indicating high level of sensitivity. In contrast, Gardner20 reported a relatively low sensitivity of 18% to 55% for exudate that was present on a dry gauze after it was placed on an ulcer for 1 hour. Results may be affected by the lack of standardized criteria to determine how much exudate was present on the dry gauze. The specificity was comparable between the two studies.
The granulation tissue within an infected wound may be friable and bleed easily due to bacterial stimulation of vascular endothelial growth factor (VEGF), resulting in excess formation of abundant but weak blood vessels.40,41 Red friable granulation tissue has been identified by Gardner20 as an indicator of infection in chronic wounds (n = 36), with a sensitivity and specificity of 82% and 76%, respectively (see Table 5). Although the operational definitions were similar, the current study estimated the sensitivity and specificity to be 45% and 85%, respectively.
 Dead and devitalized tissue acts as a pro-inflammatory nidus or bacterial growth media and can encourage bacterial proliferation.42 Research to support the relationship between the presence of debris and wound infection is scanty; the current study demonstrated a significant odds ratio, sensitivity, and specificity of 5.63 (CI = 2.19 to 14.45), 62%, and 78%, respectively.
Dead and devitalized tissue acts as a pro-inflammatory nidus or bacterial growth media and can encourage bacterial proliferation.42 Research to support the relationship between the presence of debris and wound infection is scanty; the current study demonstrated a significant odds ratio, sensitivity, and specificity of 5.63 (CI = 2.19 to 14.45), 62%, and 78%, respectively.
The presence of a foul odor is associated with anaerobic and some Gram-negative (eg, Pseudomonas) bacteria; putrid discharge is attributed to products of bacterial metabolism including volatile fatty acids (propionic, butyric, valeric, isobutyric, and isovaleric acids), volatile sulfur compounds, putrescine, and cadaverine.42 The presence of odor may not be a sensitive sign according to a systematic review of 15 studies44 indicating that odor is noted in only about half of chronic wounds infected with anaerobes. In the current study, the strength of the association between smell and wound infection was significant, with an odds ratio of 3.59 (CI = 1.22 to 10.58). The estimated specificity was 86% and sensitivity was only 37%, suggesting that smell may be difficult to detect.
Wound size may increase due to an excessive amount of MMPs produced by a heavy bacterial burden. Certain bacteria have the ability to produce specific proteolytic enzymes such as invasins that attack protein and extracellular matrix component.45 This type of wound enlargement needs to be distinguished from the failure to correct the underlying cause of the wound such as lack of compression therapy in a person with venous leg edema or lack of plantar pressure redistribution and loss of protective sensation with neuropathy in a person with diabetes. To date, this is the first study to validate the use of increase in wound size as a criterion to determine bacterial burden. The odds ratio, sensitivity, and specificity were 5.00 (1.82 to 13.76), 50%, and 83%, respectively.
Using the back of the hand to detect elevated temperature of the surrounding skin may not be reliable (ie, renders a low sensitivity). In other validation studies, Gardner20 and Greenwald et al23 documented that inter-observer agreement was poor for warmth as a criterion to assess wound infection (kappa = 0.48). To enhance accuracy, infrared thermometry has been introduced to detect objective changes in skin temperature. In a clinical trial, Armstrong et al46 reported a temperature difference of 2.81o F ± 5.75o F between diabetic foot ulcers of the infected limb and the corresponding site on the contralateral foot. In the present study, wounds with an elevated temperature using infrared thermometry were eight times more likely to be diagnosed with deep infection.
Using probing-to-bone tests in diabetic foot ulcer patients, Grayson et al47 demonstrated a sensitivity of 66% and a specificity of 86% for osteomyelitis confirmed by bone biopsies (n = 50). In a similar clinical trial, Lavery et al48 confirmed the probing-to-bone test was sensitive (0.87) and specific (0.91). In a recent review, Butalia et al49 concluded osteomyelitis was six times more likely to occur when diabetic ulcers were probed to bone. Considering all types of chronic wounds, the probing to bone test has a sensitivity and specificity of 40% and 81%, respectively, for wound infection in this analysis. Results were similar to findings reported in the literature.
Previous studies indicated that local erythema was difficult to detect: Greenwald et al,23 in a study of 115 chronic wounds, reported a kappa of 0.48, indicating poor inter-rater agreement. Lorentzen and Gottrup28 recruited six wound care specialists to assess 120 nonhealing wounds and documented that the sensitivity ranged from .34 to .91. In the original conceptualization (STONES), the three E’s — edema, erythema, and exudate — were combined together as one indicator. The current analysis suggests that exudate alone was a significant (odds ratio 4.13, CI = 1.72 to 9.91) indicator of increased bacterial count. However, edema and erythema individually were insignificant predictors of bacterial damage but in combination the odds ratio was 4.88 (CI = 1.79 to 13.27); associated sensitivity and specificity were 87% and 44%. Erythema and edema were also indicators of periwound inflammation (eg, from physical trauma, chemical irritation, and skin stripping) that may have affected their individual values as indicators for wound infection.
The findings indicate that some signs may be more valid than others for the evaluation of bacterial damage in chronic wounds. However, no one sign can be considered the most definitive or accurate. To determine how a combination of signs may help evaluate bacterial burden in wounds, sensitivity and specificity were calculated for wounds that exhibited any of the two, three, or four signs (see Table 4). Results suggest that the use of any three signs provides a valid and practical approach to determine the presence and level of bacterial damage.
Limitations
Although infection can trigger an inflammatory response in patients with chronic wounds, the proposed signs of infection also may be observed in other inflammatory conditions such as recurrent trauma, deep structure injury, vasculitis, or pyoderma gangrenosum.50 The potential confounding effect of these inflammatory conditions on the results of this study cannot be excluded. Additional patient factors such as impaired circulation, medications, and other comorbidities that may affect healing, local temperature, exudate production and bacterial contamination were not controlled in this study. In addition, both clinicians conducting the assessments were experts and familiar with the mnemonic. Content validity and reliability studies using other expert or nonexpert clinicians have not been conducted. Finally, because patients were assessed only once, neither the predictive value of the observed bacterial counts nor that of the assessment variables can be ascertained.
Conclusion
The diagnosis of infection should be made clinically. In general, a bacterial swab and culture will identify antimicrobial agent sensitivity as well as the presence of multi-resistant organisms.
The results of this study seem to confirm that no one clinical sign is sufficient to indicate the presence or amount of bacteria in the wound. By extension, no one clinical sign may be sufficient to help clinicians decide whether a topical or systemic antibiotic should be prescribed. The mnemonic NERDS© and STONEES© divides signs for superficial critical colonization and deep or surrounding skin infection into two clusters of any three or more signs with the overlap of increased exudate and odor in both groups. In the current study, any three criteria were found to provide sensitive and specific information about the amount of bacteria present in the wound when used by expert clinicians familiar with the mnemonic. Content validity and reliability studies using expert and nonexpert clinicians as well as studies to examine the relationship between these assessment variables, bacterial counts, and clinical outcomes are needed.
Dr. Woo is a Clinical Scientist/Wound Care Specialist, Wound Healing Clinic, Women’s College Hospital, Women’s College Research Institute, Toronto, Ontario, Canada; and Public Health Science, University of Toronto. Dr. Sibbald is the Medical Director, Wound Healing Clinic, Women’s College Hospital, Toronto, Ontario, Canada; and Public Health Science, University of Toronto.
1. Bowler PG, Davies BJ. The microbiology of infected and noninfected leg ulcers. Int J Dermatol. 1999;38:573–578.
2. Landis S, Ryan S, Woo K, et al. Infections in chronic wounds. In: Krasner DL, Rodeheaver GT, Sibbald RG. Chronic Wound Care: A Clinical Source Book for Healthcare Professionals, 4th ed. Malvern, PA: HMP Communications;2007:99–321.
3. Frank C, Bayoumi I, Westendorp C. Approach to infected skin ulcers. Can Family Phys. 2005;51:1352–1359.
4. Davies CE, Hill KE, Newcombe RG, et al. A prospective study of the microbiology of chronic venous leg ulcers to reevaluate the clinical predictive value of tissue biopsies and swabs. Wound Rep Reg. 2007;15:17–22.
5. Bendy RH, Nuccio PA, Wolfe E, et al. Relationship of quantitative wound bacterial counts to healing of decubiti: effect of topical gentamicin. Antimicrob Agents Chemother. 1964;10:147–155.
6. Ovington LG. Bacterial toxins and wound healing. Ostomy Wound Manage. 2003;49(7a suppl):8–12.
7. Liu Y, Min D, Bolton T. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabet Care. 2009;32(1):117-119.
8. Muller M, Troome C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet Med. 2008;25(4):419–426.
9. Mwaura B, Mahendran B, Hynes N. The impact of differential expression of extracellular matrix metalloproteinase inducer, matrix metalloproteinase-2, tissue inhibitor of matrix metalloproteinase-2 and PDGF-AA on the chronicity of venous leg ulcers. Eur J Vasc Endovasc Surg. 2006;31(3):306–310.
10. Woo K, Ayello EA, Sibbald RG. The edge effect: current therapeutic options to advance the wound edge. Advances Skin Wound Care. 2007;20:99–117.
11. Branom R. Is this wound infected? Wound and skin management in the ICU. Crit Care Nurs Quart. 2002;25(1):55–62.
12. Williams DT, Hilton JR, Harding KG. Diagnosing foot infection in diabetes. CID. 2004;39(2 suppl ):S83–S86.
13. Danilla S, Andrades P, Gomez ME, et al. Concordance between qualitative and quantitative cultures in burned patients analysis of 2,886 cultures. Burns. 2005;31:967–971.
14. Gardner SE, Frantz RA, Saltzman CL, et al. Diagnostic validity of three swab techniques for identifying chronic wound infection. Wound Rep Reg. 2006;14:548–557.
15. Slater RA, Lazarovitch T, Boldur I, et al. Swab cultures accurately identifying bacterial pathogens in diabetic foot wounds not involving bone. Diabet Med. 2004;21:705–709.
16. Pellizzer G, Strazzabosco M, Presi S, et al. Deep tissue biopsy vs. superficial swab culture monitoring in the microbiological assessment of limb-threatening diabetic foot infection. Diabet Med. 2001;18: 822–827.
17. Cutting KF, Harding KG. Criteria for identifying wound infection. J Wound Care. 1994;3:198–201.
18. Cutting KF. Identification of infection in granulating wounds by registered nurses. J Clin Nurs. 1998;7(6):539–546.
19. Cutting KF, White RJ. Criteria for identifying wound infection revisited. Ostomy Wound Manage. 2005;51(1):28–34.
20. Gardner SE. The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Rep Reg. 2001;9:178–186.
21. Prompers L, Huijberts M, Apelqvist J, et al. High prevalence of ischemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50:18–25.
22. Serena T, Robson MC, Cooper DM, et al. Lack of reliability of clinical/visual assessment of chronic wound infection: the incidence of biopsy proven infection in venous leg ulcers. Wounds. 2006;18(7):197–202.
23. Greenwald PW, Schaible DD, Ruzich JV, Prince SJ, Birnbaum AJ, Bijur PE. Is single observer identification of wound infection a reliable endpoint? J Emerg Med. 2002;23(4):333–335.
24. Wilson AP, Gibbons C, Reeves BC, et al. Surgical wound infection as a performance indicator: agreement of common definitions of wound infection in 4,773 patients. BMJ. 2004;329(7468):720.
25. Sheretz RJ, Garibaldi RA, Marosok RD. Consensus paper on the surveillance of surgical site infections. Am J Infect Control. 1992;20:263–270.
26. Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections. Infect Control Hosp Epidemiol. 1992;13(10):606–608.
27. Wilson AP, Treasure T, Sturridge MF, Gruneberg RN. A scoring method (ASEPSIS) for postoperative wound infections for use in clinical trials of antibiotic prophylaxis. Lancet. 1986;1:311–313.
28. Lorentzen HF, Gottrup F. Clinical assessment of infection in non-healing ulcers analyzed by latent class analysis. Wound Rep Reg. 2006;14:350–353.
29. Sibbald RG, Woo K, Ayello EA. Increased bacterial burden and infection: the story of NERDS and STONES. Adv Skin Wound Care. 2006;19(8):447–461.
30. van Rijswijk L, Catanzaro J. Wound assessment and documentation. In: Krasner DL, Rodeheaver GT, Sibbald RG. Chronic Wound Care: A Clinical Source Book for Healthcare Professionals, 4th ed. Malvern, PA: HMP Communications;2007:113-126.
31. Ratliff CR, Rodeheaver GT. Correlation of semi-quantitative swab cultures to quantitative swab cultures from chronic wounds. Wounds. 2002;14:329–333.
32. Bouza E, Burillo A, Munoz P, Cercenado E, Rodriquez-Creixems M. Semiquantitative culture of open surgical wounds for diagnosis of surgical site infection. Eur J Clin Microbiol Infect Dis. 2004;23(2):119–122.
33. Landes SJ. Chronic wound infection and antimicrobial use. Adv Skin Wound Care. 2008;21(11):531–540.
34. Sarvis CM. Calling on NERDS for critically colonized wounds. Nursing. 2007;37(5):26–27.
35. Miller R. Puzzling cases: non-healing venous leg ulcer. Wound Care Canada. 2006;4(3):38.
36. Elahi MM, Haesey AM, Graham KC, et al. Leg wound infections following cardiac surgery: a scoring system for assessment and management. J Wound Care. 2005;14:337–340.
37. Meaume S, Vallet D, Morere MN, Teot L. Evaluation of a silver-releasing hydroalginate dressing in chronic wounds with signs of local infection. J Wound Care. 2005;14(9):411–419.
38. Okan D, Woo K, Ayello EA, Sibbald RG. The role of moisture balance in wound healing. Adv Skin Wound Care. 2007;20(1):39–53.
39. Woo KY, Harding K, Price P, Sibbald RG. Minimising wound-related pain at dressing change: evidence-informed practice. Int J Wound. 2008;5(2):144–157.
40. Sen CK, Khanna S, Babior BM, Hunt TK, Ellison EC, Roy S. Oxidant-induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J Biol Chem. 2002;277(36):33284–33290.
41. Svendsen MN, Lykke J, Werther K, Bisgaard T, Christensen IJ, Nielsen HJ. Bacterial antigen induced release of soluble vascular endothelial growth factor (VEGF) and VEGFR1 before and after surgery. Scand J Clin Lab Invest. 2005;65(3):237–247.
42. Beitz JM. Wound debridement: therapeutic options and care considerations. Nurs Clin North Am. 2005;40:233–249.
43. Hapmson JP. The use of metronidazole in the treatment of malodorous wounds. J Wound Care. 1996;5(9):421–425.
44. Paul JC, Pieper BA. Topical metronidazole for the treatment of wound odor: a review of the literature. Ostomy Wound Manage. 2008;54(3):18–27.
45. Cambronne ED, Schneewind O. Bacterial invasions: molecular systems dedicated to the invasion of host tissues. Contrib Microbiol. 2005;12:181–209.
46. Armstrong DG, Lipsky BA, Polis MB, et al. Does dermal thermometry predict clinical outcome in diabetic foot infection? Analysis of data from the sidestep trial. Int Wound J. 2006;3(4):302¬–307.
47. Grayson ML, Gibbons GW, Balogh K, et al. Probing to the bone in infected pedal ulcers: a clinical sign of underlying osteomyelitis in diabetic patients. JAMA. 1995;273(9):721–723.
48. Lavery LA, Armstrong DG, Peters EJG, et al. Probe to the bone test for diagnosing diabetic foot osteomyelitis. Reliable or relic? Diabetes Care. 2007;30(2):270–274.
49. Butalia S, Palda VA, Sargeant RJ. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA. 2008;299(7):806–813.
50. Sibbald RG, Orsted H, Schultz GS, Coutts P, Keast D, International Wound Bed Preparation Advisory Board. Preparing the wound bed 2003: focus on infection and inflammation. Ostomy Wound Manage. 2003;49(11):23–51.













