Efficacy of a Bio-electric Dressing in Healing Deep, Partial-thickness Wounds Using a Porcine Model
Abstract
Numerous physical modalities have been used in attempts to augment the healing process, including ultrasound, low-energy light therapy, and electrical stimulation (ES). ES has been shown to benefit tissue repair in a variety of wound types, but variations in study designs, administration, and parameters render its application in clinical practice somewhat unconventional. A dressing was designed to generate an electric potential of 0.6 V to 0.7 V in the presence of moisture, thereby delivering a sustained micro-current without the need for an external power source. The purpose of this study was to examine the effects of this bio-electric dressing (BED) on deep, partial-thickness wounds using six female specific pathogen-free animals and a well established porcine model for wound healing. Wounds (10 mm x 7 mm x 0.5 mm) were created in paravertebral and thoracic areas of these animals using a specialized electrokeratome and covered with the active polyester BED and a polyurethane film dressing (n = 30) (treatment) or an inactive polyester and film dressing (n = 30). Using an epidermal migration assay, wounds were assessed daily from day 4 through day 8 post-wounding. Differences in the proportion of wounds healed were statistically significant (P <0.001) on days 5 and 6 post-wounding. These results show BED is more effective than a control dressing treatment with moisture-retentive dressings in this animal model. Controlled clinical studies are warranted to elucidate the potential clinical implications of this treatment modality. {C}
Potential Conflicts of Interest: Monetary compensation was received from Silverleaf, Inc (now Vomaris, Inc, Chandler, AZ) to conduct the experiment. No one participating in the creation, execution, or analysis of this study has any commercial, proprietary, or financial interest in the success of the product.
Introduction
Wound healing consists of a series of biological processes in which numerous cells form a response to an injury that results in tissue deposition, re-epithelialization, and scar formation.1 An important mechanism involved is the electric potential produced naturally in the skin that aids in the recruitment of cells necessary for healing through a process called galvanotaxis or electrotaxis.2 In attempts to augment galvanotaxis, scientists and clinicians have been experimenting with exogenous electrical stimulation (ES) as a potential wound treatment since the 1600s.3 Many studies have shown a decrease in wound contraction time, an increase in wound tensile strength, and a reduction of scarring through a variety of ES delivery methods. In 1968, Assimacopoulous4 witnessed a 25% faster healing rate within full-thickness wounds in rabbit ears treated with continuous direct current through a negative electrode compared to a control group. Treatment of full-thickness wounds in vivo with continuous negative current also has been shown to increase tensile strength by 53% compared to wounds treated with a sham generator.5 A study involving the treatment of partial-thickness wounds in the paravertebral region of pigs using positive-polarity direct current resulted in increased epithelialization and collagen deposition when compared to untreated wounds.6 Using high-voltage pulsed ES for 45 minutes a day, Kloth and Feedar7 were able to heal the decubitus ulcers of three patients at a mean rate of 44.8% per week; in the three patients who served as controls for the study and received no stimulation from the electrodes, ulcers increased in area by 11.6% per week. Although no mechanism had yet been described for ES’s effects on the healing process when these studies were conducted, such positive results gave reason for future research and development of ES as a wound therapy.
Prior investigation has demonstrated that the skin contains an electronegative gradient with the stratum corneum usually having an increased negative charge in comparison to the dermis. The difference in charge produces an electropotential of 30 mV to 100 mV across the epidermis.8 In order to maintain this gradient, epithelial cells actively transport sodium ions between themselves through tight junctions.9 When these cells break down due to injury, the surrounding undamaged skin holds a measurable negative charge with respect to the exposed dermis, and an electrical gradient is noted at the skin’s surface.10,11 A flow of surface charge creates a low-intensity direct current (DC) that has been measured to range from 10 µA/cm² to 30 µA/cm² as the distance from the site of injury decreases.5
A myriad of results from both in vivo and clinical wound healing trials corroborate ES’s viability as a wound healing treatment. In comparison to nontreated wounds, Mertz et al12 described a 20% decrease in healing time for wounds treated with 1 day of negative polarity followed by successive days of positive polarity in a porcine model. A clinical trial13 involving patients presenting with comparable venous ulcers also showed results supporting the use of ES; persons treated with high-voltage pulsed current (HVPC) (n = 33) exhibited larger reductions in wound size, as well as significant increases in pus clearance and granulation tissue development compared to those treated with topical ointments (n = 32) or the Unna boot (n = 14). A more recent clinical study14 analyzing ES’s influence on edema in mixed etiology chronic leg wounds of 30 patients over a 10-day treatment period resulted in a sustained 60% reduction in edema 10 days after treatment. These data lend promising evidence for the efficacy of ES on the wound healing process, as past in vivo studies15,16 involving the knockout of genes that play roles in the inflammatory process have shown that their deletion leads to improved healing within full-thickness wounds. However, discrepancy remains over which forms of ES provide the most effective means of wound closure. This is primarily due to ambiguous study parameters that inhibit experimental reproducibility, which leaves a need for more research using standardized parameters in order to confirm the most beneficial modes of administration.17
 Another wound treatment modality with decades of scientific data is moist wound healing through occlusive dressings. By maintaining a moist wound environment, occlusive dressings have been shown in preclinical and clinical studies18,19 to expedite cell migration, sustain growth factor viability, and reduce infection rates and scar formation compared to control wounds. In animal and human studies,20-25 various dressings have been shown to increase re-epithelialization rates by up to 50% compared to controls. Some clinical studies26,27 suggest occlusive dressings reduce the inflammation and pain associated with both acute and chronic wounds. Overall, there is substantial preclinical and clinical evidence advocating for the maintenance of the appropriate moisture levels within wounds through the use of moist occlusive dressings. Such studies28,29 have demonstrated that a moist wound environment aids the healing process by facilitating epithelial cell coverage, providing a favorable environment for beneficial microbial flora, boosting the local concentration of growth factors, amplifying the partial pressure of oxygen, and sustaining the electric potential between wounded and normal skin described above.
Another wound treatment modality with decades of scientific data is moist wound healing through occlusive dressings. By maintaining a moist wound environment, occlusive dressings have been shown in preclinical and clinical studies18,19 to expedite cell migration, sustain growth factor viability, and reduce infection rates and scar formation compared to control wounds. In animal and human studies,20-25 various dressings have been shown to increase re-epithelialization rates by up to 50% compared to controls. Some clinical studies26,27 suggest occlusive dressings reduce the inflammation and pain associated with both acute and chronic wounds. Overall, there is substantial preclinical and clinical evidence advocating for the maintenance of the appropriate moisture levels within wounds through the use of moist occlusive dressings. Such studies28,29 have demonstrated that a moist wound environment aids the healing process by facilitating epithelial cell coverage, providing a favorable environment for beneficial microbial flora, boosting the local concentration of growth factors, amplifying the partial pressure of oxygen, and sustaining the electric potential between wounded and normal skin described above.
Given the growing evidence supporting the beneficial effects of both ES and moist occlusive dressings on wound healing, it would seem logical to take advantage of the relationship between a moist wound environment and the electric potential of the skin. One recently developed treatment therapy combines both modalities through the incorporation of an endogenous electric field (EF) in an occlusive wound dressing using elements similar to those found in a galvanic cell battery. The bio-electric dressing, referred to as BED in this in vivo study, is comprised of a woven polyester fabric surface containing a matrix of biocompatible elemental silver and zinc dots 2 mm and 1 mm in diameter, respectively. The contact material requires no external power source and activates in the presence of a conductive fluid, which may come from wound exudate or exogenous fluids such as normal saline, generating an electric potential of 0.6 V to 0.7 V between the silver and zinc dots. Additionally, a secondary moist occlusive dressing may be placed over the BED to maintain moisture. The result is a sustained micro-current similar to that of skin injury (data on file with company).
The purpose of this study was to examine the rate of re-epithelialization within partial-thickness wounds treated with the BED dressing compared to those treated with a traditional polyester gauze dressing using a well-established porcine model.
Materials and Methods
Experimental animals. A porcine model was used for this research due to the morphological similarities between swine skin and human skin.29 Six female specific pathogen-free pigs weighing 25 kg to 30 kg were kept in house for 2 weeks before initiating the experiment. All procedures were approved by the University of Miami’s Animal Use Committee and conducted in compliance with the University of Miami’s Department of Dermatology and Cutaneous Surgery’s Standard Operating Procedures.
Wounding technique. The flanks and backs of the experimental animals were clipped with standard animal clippers on the first day of experimentation. The skin on both sides of each animal was prepared for wounding by washing with a non-antibiotic soap (Neutrogena Soap Bar; Johnson and Johnson, Los Angeles, CA) and sterile water. After anesthetizing each pig, 60 wounds measuring 10 mm x 7 mm x 0.5 mm deep were made on the paravertebral and thoracic area with a specialized electrokeratome fitted with a 7-mm blade. The wounds were separated from one another by 15 mm of unwounded skin. To prevent any discomfort post-wounding, each animal was given time-release analgesics throughout the entire experiment.
 Treatment. Thirty wounds were randomly assigned to one of two treatment groups: 1) active BED or 2) control woven polyester material without active bio-electric components (see Figure 1). All dressings, each cut to size to fit over the entire wounded area, were saturated in sterile buffered saline before application in order to promote a moist environment and enhance the electrical conductivity within wounds. Each test and control article was individually covered with a polyurethane film dressing (3M Tegaderm™, St. Paul, MN) to further retain moisture. All wound dressings were secured using elastic tape to ensure intimate contact between the wound surface and the dressing, as well as to prevent accidental removal. Then the entire treatment area was loosely wrapped with an elastic bandage (Coban; 3M, St. Paul, MN) to prevent removal of the treatments. All dressings were replaced on days 1, 4, and 7 post-wounding (day 0). Each was moistened with sterile saline before removal to prevent re-injury of the treatment area.
Treatment. Thirty wounds were randomly assigned to one of two treatment groups: 1) active BED or 2) control woven polyester material without active bio-electric components (see Figure 1). All dressings, each cut to size to fit over the entire wounded area, were saturated in sterile buffered saline before application in order to promote a moist environment and enhance the electrical conductivity within wounds. Each test and control article was individually covered with a polyurethane film dressing (3M Tegaderm™, St. Paul, MN) to further retain moisture. All wound dressings were secured using elastic tape to ensure intimate contact between the wound surface and the dressing, as well as to prevent accidental removal. Then the entire treatment area was loosely wrapped with an elastic bandage (Coban; 3M, St. Paul, MN) to prevent removal of the treatments. All dressings were replaced on days 1, 4, and 7 post-wounding (day 0). Each was moistened with sterile saline before removal to prevent re-injury of the treatment area.
Data collection and analysis
Epidermal migration assessment. Using a well established epidermal migration assessment,22,31-33 five wounds and the surrounding normal skin from each treatment group were excised intact once daily on days 4 through 9 using an electrokeratome with a 22-mm blade set at a depth of 0.7 mm. The excised skin containing the wound site was incubated in 0.5 M sodium bromide at 37˚ C for 24 hours, allowing for a separation of the dermis from the epidermis at the basement membrane zone. After separation, the epidermal sheet was examined macroscopically for defects. Defects were defined as holes in the epidermal sheet or a lack of epidermal continuity in the area of the wound. Epithelialization was considered complete (healed) if no defect(s) was present; any aberration in the wound area indicated healing was incomplete. The mounted samples were retained for a permanent record.
After the study was completed, the number of wounds found to be completely healed (completely epithelialized) on each assessment day per treatment were combined for all six pigs. These sums then were divided by the total number of wounds excised from each treatment group in all six pigs per assessment day (n = 30) and the ratio multiplied by 100 to give the percentages of wounds healed. These percentages were plotted against days after wounding, and results of the treatment and control dressings were compared. Chi-squared tests were used to determine statistical significance of proportions healed between treatment groups. 
Results
Assessment on day 4 post-wounding showed that no wounds excised from either treatment group had completely re-epithelialized. However, by day 5, a mean of 67% of BED-treated wounds compared to a mean of 20% of the polyester-treated wounds in the control group were completely epithelialized (P <0.001). The trend continued to the following day (day 6), with 93% complete epithelialization in the BED treatment group compared to 50% in the polyester treatment control group (P <0.001). On day 7, all of the BED-treated and 87% of the polyester-treated wounds had completely healed. This difference was not statistically significant (see Figures 2 and 3). 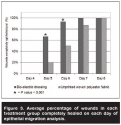
Discussion
The results from this study show that epidermal migration of deep partial-thickness wounds in this animal model occurred more expediently in wounds covered with a BED and film dressing than those covered with a control fabric and film dressing. Wounds in the treatment group experienced a rapid increase in epithelial migration between days 4 and 5, with 0% of the wounds completely epithelialized on day 4 and 67% on day 5. This healing rate was significantly higher than in those treated with the polyester control dressing. Similarly, on day 6, 93% of the treatment group wounds assessed were completely covered with epithelium compared to only 50% of the polyester treated wounds.
These results correlate with those of a clinical study34 involving the use of BED to treat split-thickness skin donor site wounds (n = 13). Patients who received the bioelectric dressing covered with a semi-occlusive dressing healed 34.62% faster than those who received a standard semi-occlusive dressing alone. These patients reported improved scar color, reduced scar thickness and stiffness, and overall superior scar quality compared to the control group. Such results, along with those from the current in vivo tests, support BED as a viable wound treatment option capable of augmenting the current of injury and the healing process.
Other in vivo and clinical trials involving variable modes of ES delivery correspond to the data generated from this porcine study. Using an energized silver-coated electrode to deliver a direct current of 50 µA to 300 µA to partial-thickness wounds in a porcine model, Alvarez et al35 produced significantly accelerated healing rates (P <0.05) compared to wounds exposed to an unenergized electrode. In another investigation,36 the delivery of 20 µA DC to 40 µA DC to deep partial-thickness burn wounds in guinea pigs through silver nylon dressings also promoted healing.
Pulsed current also has demonstrated beneficial effects. A clinical study13 involving the use of either alternating polarity of HVPC, topically applied medicine, or the Unna boot on 79 patients suffering from wounds caused by chronic venous insufficiency showed that the HVPC resulted in a 59% decrease in overall wound size as compared to the Unna boot alone, which produced a 25% reduction. These reports add considerable evidence in favor of ES’s ability to expedite wound closure.
ES mechanisms of action. Numerous in vitro tests have begun to elucidate the cellular responses to ES. Dermal fibroblasts have been observed to migrate toward the anode when exposed to an electric field of 100 mV/mm.37 Human epithelial cells treated with pulsed electromagnetic fields (EMFs) have expressed increased levels of FGF-2, a mediator that induces proliferation of fibroblasts and other cells necessary for proper healing.38 A study39 using an incisional wound model observed no effects on fibroblast movement when exposed to an electromagnetic field (EMF) (2.5 mV/cm for 1 hour), but did demonstrate an increase in migration and proliferation of keratinocytes. Similarly, keratinocytes have been witnessed to migrate toward the cathode when treated with DC ES.40-42 Other in vitro experiments have illustrated that neutrophils and macrophages also travel toward the cathode when exposed to electric fields and that the distance and speed of migration increases with increasing field strength.43,44
The mechanism by which EFs attract cells to wound sites could be primarily due to induced changes in cell morphology. Some observations have led scientists to believe that EFs cause cellular membrane depolarizations, which in turn locally activate voltage-dependent calcium (Ca²+) channels.45 Poorly differentiated keratinocytes also have been shown to exhibit a similar response to EF.46 A local influx of Ca²+ ions is possibly required for the polymerization of actin filaments that make up the cell’s cytoskeleton. Cytoskeletal remodeling thereby elongates the cell, orients it toward a direction of migration, and creates lamellipodial extensions that allow for cell movement.39,47-49 Alternatively, other data43 provide evidence that galvanotaxis is independent of actin reorganization, but rather the polarization of a group of signal transduction cascades. It is important to note that all of these observations were the results of in vitro studies. Although they indicate promising hypotheses for the underlying mechanisms of EF induced cell migration, it is difficult to know whether such phenomenon occur in vivo.
Bio-electric dressing. The studies conducted to date suggest that the results of BED treatment are similar to those reported using other various forms of ES. BED’s attractiveness as a wound care product lies in its design. By combining the beneficial wound repair characteristics of both an occlusive dressing and an electrical gradient, it simultaneously utilizes two separate modalities that have been shown to aid wound healing. Other classical methods of ES usually require separate occlusive dressings in addition to electrodes that create the flow of current, a treatment design that prevents the patient from receiving a constant electrical current similar to that produced naturally within the wound site. Furthermore, electrodes require an outside power source, making it necessary for the patient to remain immobile for the treatment period. However, BED can remain on the wound site for continued delivery of ES as long as it remains moist (via endogenous or exogenous fluids). Because the entire treatment is contained in a single material, it can be applied and changed easily without the required presence of someone specially trained in ES. Thus, wounds can continuously receive the combined benefits of constant ES while maintaining a moist wound environment in an outpatient setting.
Conclusion
The increased proportion of healed deep partial-thickness wounds with the BED compared to the control wounds suggests that the bio-electric qualities of the treatment may supplement the endogenous “skin battery” and promote the galvanotaxis and proliferation of cells required for healing. In order to elucidate further information regarding this hypothesis, it would be interesting to measure the electric potential of the skin and the levels of assorted mediators responsible for the activity of various cells. Moreover, the results from both this porcine study and the aforementioned skin graft donor site clinical trial establish the need for additional clinical trials. Specifically, studies are needed to determine the efficacy of BEDs on various wound types (eg, acute, chronic, burn) in comparison to existing ES devices, as well as standard therapies. If results of future studies confirm the encouraging observations reported here, patients may benefit from a user-friendly option of receiving ES to facilitate the healing process.
Mr. Harding is a research assistant; Mr. Gil is a research associate; Mr. Valdes is a senior research assistant; Mr. Solis is a research assistant; and Mr. Davis is a research professor, University of Miami Miller School of Medicine, Miami, FL. Please address correspondence to: Stephen C. Davis, Research Professor, University of Miami Miller School of Medicine, Department of Dermatology & Cutaneous Surgery, 1600 N.W. 10th Ave. Room 2089. Miami, FL 33136; email: sdavis@med.miami.edu..
1. Broughton G II Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 suppl):1e-S–32e-S.
2. Tai G, Reid B, Cao L, Zhao M. Electrotaxis and wound healing: experimental methods to study electric fields as a directional signal for cell migration. Methods Mol Biol. 2009;571:77–97.
3. Robertson WS. Digby’s receipts. Ann Med Hist. 1925;7(3):216–219.
4. Assimacopoulos D. Wound healing promotion by the use of negative electric current. Am Surg. 1968;34:423–431.
5. Konikoff JJ. Electrical promotion of soft tissue repairs. Ann Biomed Eng. 1976;41:1–5.
6. Alvarez OM, Mertz PM, Smerbeck RV, Eaglstein WH. The healing of superficial skin wounds is stimulated by external electrical current. J Invest Dermatol. 1983;81:144–148.
7. Kloth LC, Feedar JA. Acceleration of wound healing with high voltage, monophasic pulsed current. Phys Ther. 1988;68:503–508.
8. Barker AT, Jaffe LF, Vanable JW Jr. The glabrous epidermis of cavies contains a powerful battery. Am J Physiol. 1982;242:R358–366.
9. Klyce SD. Electrical profiles in the corneal epithelium. J Physiol. 1972;226(2):407–429.
10. McCaig CD, Robinson KR. The ontogeny of the transepidermal potential difference in frog embryos. Dev Biol. 1982;90(2):335–339.
11. Talebi G, Torkaman, G, Firoozabadi M, Shariat S. Effect of anodal and cathodal microamperage direct current electrical stimulation on injury potential and wound size in guinea pigs. J Rehabil Res Dev. 2008;45(1):153–159.
12. Mertz PM, Davis SC, Cazzaniga AL, Cheng K, Reich JD, Eaglstein WH. Electrical stimulation: acceleration of soft tissue repair by varying the polarity. WOUNDS. 1993;5(3):153–159.
13. Franek A, Polak A, Kucharzewski M. Modern application of high voltage stimulation for enhanced healing of venous crural ulceration. Med Eng Phys. 2000;22(9):647–655.
14. Young S, Hampton S, Tadej M. Study to evaluate the effect of low-intensity pulsed electrical currents on levels of oedema in chronic non-healing wounds. J Wound Care. 2011;20(8):368–363.
15. Ashcroft GS, Yang X, Glick AB, et al. Mice laking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266.
16. Outtz HH, Wu JK, Wang X, Kitajewski J. Notch 1 deficiency results in decreased inflammation during wound healing and regulates vascular endothelial growth factor receptor-1 and inflammatory cytokine expression in microphages. J Immunol. 2010;185(7):4363–4373
17. Reich JD, Tarjan PP. Electrical stimulation of skin. Int J Dermatol. 1990;29(6):395–400.
18. Hermans M. An overview of physiological aspects of occlusive and non-occlusive dressings. Primary Intention. 1995;3:8–13.
19. Mertz PM, Marshsll DA, Eaglstein WH. Occlusive wound dressings to prevent bacterial invasion and wound infection. J Am Acad Dermatol. 1985;12(4):662–668.
20. Winter GD. Formation of scab and the rate of epithelialization of superficial wounds in the skin of the domestic pig. Nature. 1962;193:293–294.
21. Hinman CC, Maibach H, Winter GD. Effect of air exposure and occlusion on experimental human skin wounds. Nature. 1963;200:377–378.
22. Eaglstein WH, Mertz PM. New methods for assessing epidermal wound healing: the effects of triamcinolone acetonide and polyethelene film occlusion. J Invest Dermatol. 1978;71(6):382–384.
23. Alvarez OM, Mertz PM, Eaglstein WH. The effect of occlusive dressings on collagen synthesis and re-epithelialization in superficial wounds. J Surg Res. 1983;35(2):142–148.
24. Harris B, Cai JP, Falanga V, Mertz P, Chin YH, Eaglstein W. The effects of occlusive dressings on the recruitment of mononuclear cells by endothelial binding into acute wounds. J Dermatol Surg Oncol. 1992;18(4):279–283.
25. De Coninck A, Draye JP, Van Strubarg A, Vanpée E, Kaufman L, Delaey B, Verbeken G, Roseeuw D. Healing of full-thickness wounds in pigs: effects of occlusive and non-occlusive dressings associated with a gel vehicle. J Dermatol Sci. 1996;13(3):202–211.
26. van Rijswijk L, Brown D, Friedman S, et al. Multicenter clinical evaluation of a hydrocolloid dressing for leg ulcers. Cutis. 1985;35(2):173–176.
27. Mabrouk A, Boughdadi NS, Helal HA, Zaki BM, Maher A. Moist occlusive dressing (Aquacel(®)Ag) versus moist open dressing (MEBO®) in the management of partial-thickness facial burns: a comparative study in Ain Shams University. Burns. 2011;doi:10.1016/j.burns.2011.09.022.
28. Wiechula R. The use of moist wound-healing dressings in the management of split-thickness skin graft donor sites: a systematic review. Int J Nurs Pract. 2003;9(2):S9–S17.
29. Eaglstein WH. Effects of occlusive dressings on wound healing. Clin Dermatol. 1984;2:107–111.
30. Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001;9(2):66–76.
31. Eaglstein WH, Davis SC, Mehle AL, Mertz PM. Optimal use of an occlusive dressing to enhance healing. Effect of delayed application and early removal on wound healing. Arch Dermatol. 1988;124(3):392–395.
32. Kaiser MR, Davis SC, Mertz BA. Effect of ultraviolet radiation-induced inflammation on epidermal wound healing. Wound Repair Regen, 1995;3(3):311–315.
33. Davis SC, Cazzaniga AL, Ricotti C, et al. Topical oxygen emulsion: a novel wound therapy. Arch Dermatol. 2007;143(10):1252–1256.
34. Blount AL, Foster S, Rapp DA, Wilcox R. The use of bioelectric dressings in skin graft harvest sites: a prospective case series. J Burn Care Res. 2012;33(3):354–357.
35. Alvarez OM, Mertz PM, Smerbeck RV, Eaglstein WH. The healing of superficial skin wounds is stimulated by external electrical current. J Invest Dermatol. 1983;81(2):144–148.
36. Chu CS, McManus A, Mason A Jr. Okerberg C, Pruitt B Jr, Multiple graft harvestings from deep partial-thickness scald wounds healed under the influence of weak direct current. J Trauma. 1990;30(8):1044–1049.
37. Guo A, Song B, Reid B, Gu Y, Forrester JV, Jahoda CA, Zhao M. Effects of physiological electric fields on migration of human dermal fibroblasts. J Invest Dermatol. 2010;130(9):2320–2327.
38. Callaghan MJ, Chang EI, Seiser N, et al. Pulsed electromagnetic fields accelerate normal and diabetic wound healing by increasing endogenous FGF-2 release. Plast Reconstr Surg. 2008;121:130–141.
39. Huo R, Ma Q, Wu JJ, et al Noninvasive electromagnetic fields on keratinocyte growth and migration. J Surg Res. 2010;162(2):299–307.
40. Luther PW, Peng HB, Lin J, Changes in cell shape and actin distribution induced by constant electric fields. Nature. 1983;303(5912):61–64.
41. Soong HK, Parkinson WC, Bafna S, Sulik GL, Huang SC. Movements of cultured corneal epithelial cells and strombal fibroblasts in electric fields. Invest Ophthalmol Vis Sci. 1990;31(11):2278–2282.
42. Zhao M, Agious-Fernandez A, Forrester JV, McCaig, CD. Orientation and directed migration of cultured corneal epithelial cells in small electric fields are serum dependent. J Cell Sci. 1996;109:1405–1414.
43. Orida N, Feldman JD. Directional protrusive pseudopodial activity and motility in macrophages induced by extracellular electric fields. Cell Motil. 1982;2(3):243–255.
44. Zhao M, Song B, Pu J, et al. Electric signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442(7101):457–460.
45. Bedlack RS Jr, Wei M, Loew LM. Localized membrane depolarizations and localized calcium influx during electric field-guided neurite growth. Neuron. 1992;9(3):393–403.
46. Dubé J, Rochette-Drouin O, Lévesque P, et al. Human keratinocytes respond to direct current stimulation by increasing intracellular calcium: preferential response of poorly differentiated cells. J Cell Physiol. 2011. doi:10.1002/jcp.23008.
47. Onuma EK, Hui SW. Electric field-induced cell shape changes, displacement, and cytoskeletal reorganization are calcium dependent. J Cell Biol. 1988;106:2067–2075.
48. Zhao M, Bai H, Wang E, Forrester JV, McCaig CD. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci. 2004;117(Pt 3):397–405.
49. Choi H, Cho JS, Park IH, Yoon HG, Lee HM. Effects of microelectrical current on migration of nasal fibroblasts. Am J Rhinol Allergy. 2011;25(3):157–162.













